Nonreciprocal homeostatic compensation in Drosophila potassium channel mutants
- PMID: 28298298
- PMCID: PMC5454467
- DOI: 10.1152/jn.00002.2017
Nonreciprocal homeostatic compensation in Drosophila potassium channel mutants
Abstract
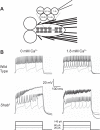
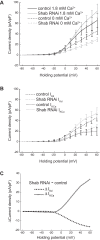
Homeostatic control of intrinsic excitability is important for long-term regulation of neuronal activity. In conjunction with many other forms of plasticity, intrinsic homeostasis helps neurons maintain stable activity regimes in the face of external input variability and destabilizing genetic mutations. In this study, we report a mechanism by which Drosophila melanogaster larval motor neurons stabilize hyperactivity induced by the loss of the delayed rectifying K+ channel Shaker cognate B (Shab), by upregulating the Ca2+-dependent K+ channel encoded by the slowpoke (slo) gene. We also show that loss of SLO does not trigger a reciprocal compensatory upregulation of SHAB, implying that homeostatic signaling pathways utilize compensatory pathways unique to the channel that was mutated. SLO upregulation due to loss of SHAB involves nuclear Ca2+ signaling and dCREB, suggesting that the slo homeostatic response is transcriptionally mediated. Examination of the changes in gene expression induced by these mutations suggests that there is not a generic transcriptional response to increased excitability in motor neurons, but that homeostatic compensations are influenced by the identity of the lost conductance.NEW & NOTEWORTHY The idea that activity-dependent homeostatic plasticity is driven solely by firing has wide credence. In this report we show that homeostatic compensation after loss of an ion channel conductance is tailored to identity of the channel lost, not its properties.
Keywords: Drosophila; excitability; homeostasis; ion channel; motor neuron.
Copyright © 2017 the American Physiological Society.
Figures



Similar articles
-
Pre- and post-synaptic mechanisms of synaptic strength homeostasis revealed by slowpoke and shaker K+ channel mutations in Drosophila.Neuroscience. 2008 Jul 17;154(4):1283-96. doi: 10.1016/j.neuroscience.2008.04.043. Epub 2008 May 2. Neuroscience. 2008. PMID: 18539401 Free PMC article.
-
Transient BK outward current enhances motoneurone firing rates during Drosophila larval locomotion.J Physiol. 2015 Nov 15;593(22):4871-88. doi: 10.1113/JP271323. Epub 2015 Oct 2. J Physiol. 2015. PMID: 26332699 Free PMC article.
-
Distinct roles of Drosophila cacophony and Dmca1D Ca(2+) channels in synaptic homeostasis: genetic interactions with slowpoke Ca(2+) -activated BK channels in presynaptic excitability and postsynaptic response.Dev Neurobiol. 2014 Jan;74(1):1-15. doi: 10.1002/dneu.22120. Epub 2013 Oct 7. Dev Neurobiol. 2014. PMID: 23959639 Free PMC article.
-
High-conductance potassium channels of the SLO family.Nat Rev Neurosci. 2006 Dec;7(12):921-31. doi: 10.1038/nrn1992. Nat Rev Neurosci. 2006. PMID: 17115074 Review.
-
Homeostatic control of neural activity: a Drosophila model for drug tolerance and dependence.Int Rev Neurobiol. 2011;99:23-50. doi: 10.1016/B978-0-12-387003-2.00002-1. Int Rev Neurobiol. 2011. PMID: 21906535 Free PMC article. Review.
Cited by
-
Dual separable feedback systems govern firing rate homeostasis.Elife. 2019 Apr 11;8:e45717. doi: 10.7554/eLife.45717. Elife. 2019. PMID: 30973325 Free PMC article.
-
A Meta-Analysis of Bioelectric Data in Cancer, Embryogenesis, and Regeneration.Bioelectricity. 2021 Mar 1;3(1):42-67. doi: 10.1089/bioe.2019.0034. Epub 2021 Mar 16. Bioelectricity. 2021. PMID: 34476377 Free PMC article.
-
Minimal requirements for a neuron to coregulate many properties and the implications for ion channel correlations and robustness.Elife. 2022 Mar 16;11:e72875. doi: 10.7554/eLife.72875. Elife. 2022. PMID: 35293858 Free PMC article.
-
Regenerative Adaptation to Electrochemical Perturbation in Planaria: A Molecular Analysis of Physiological Plasticity.iScience. 2019 Dec 20;22:147-165. doi: 10.1016/j.isci.2019.11.014. Epub 2019 Nov 9. iScience. 2019. PMID: 31765995 Free PMC article.
-
Rewiring Endogenous Bioelectric Circuits in the Xenopus laevis Embryo Model.Methods Mol Biol. 2021;2258:93-103. doi: 10.1007/978-1-0716-1174-6_7. Methods Mol Biol. 2021. PMID: 33340356
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

