How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait
- PMID: 28298343
- PMCID: PMC5360911
- DOI: 10.1098/rspb.2016.1727
How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait
Abstract
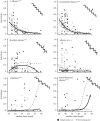
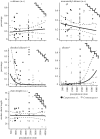
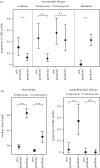
Cuticular hydrocarbons (CHCs) cover the cuticles of virtually all insects, serving as a waterproofing agent and as a communication signal. The causes for the high CHC variation between species, and the factors influencing CHC profiles, are scarcely understood. Here, we compare CHC profiles of ant species from seven biogeographic regions, searching for physiological constraints and for climatic and biotic selection pressures. Molecule length constrained CHC composition: long-chain profiles contained fewer linear alkanes, but more hydrocarbons with disruptive features in the molecule. This is probably owing to selection on the physiology to build a semi-fluid cuticular layer, which is necessary for waterproofing and communication. CHC composition also depended on the precipitation in the ants' habitats. Species from wet climates had more alkenes and fewer dimethyl alkanes than those from drier habitats, which can be explained by different waterproofing capacities of these compounds. By contrast, temperature did not affect CHC composition. Mutualistically associated (parabiotic) species possessed profiles highly distinct from non-associated species. Our study is, to our knowledge, the first to show systematic impacts of physiological, climatic and biotic factors on quantitative CHC composition across a global, multi-species dataset. We demonstrate how they jointly shape CHC profiles, and advance our understanding of the evolution of this complex functional trait in insects.
Keywords: adaptation; climatic niche; cuticular hydrocarbons; selection pressure; viscosity; water loss rate.
© 2017 The Author(s).
Figures
Similar articles
-
The evolution of a complex trait: cuticular hydrocarbons in ants evolve independent from phylogenetic constraints.J Evol Biol. 2017 Jul;30(7):1372-1385. doi: 10.1111/jeb.13115. Epub 2017 Jun 10. J Evol Biol. 2017. PMID: 28485028
-
Tolerance requires the right smell: first evidence for interspecific selection on chemical recognition cues.Evolution. 2012 Mar;66(3):896-904. doi: 10.1111/j.1558-5646.2011.01489.x. Epub 2011 Nov 18. Evolution. 2012. PMID: 22380448
-
Influence of Mutualistic Lifestyle, Mutualistic Partner, and Climate on Cuticular Hydrocarbon Profiles in Parabiotic Ants.J Chem Ecol. 2019 Sep;45(9):741-754. doi: 10.1007/s10886-019-01099-9. Epub 2019 Aug 27. J Chem Ecol. 2019. PMID: 31456059
-
Evolution of Cuticular Hydrocarbons in the Hymenoptera: a Meta-Analysis.J Chem Ecol. 2015 Oct;41(10):871-83. doi: 10.1007/s10886-015-0631-5. Epub 2015 Sep 26. J Chem Ecol. 2015. PMID: 26410609 Free PMC article. Review.
-
A review of ant cuticular hydrocarbons.J Chem Ecol. 2009 Oct;35(10):1151-61. doi: 10.1007/s10886-009-9695-4. Epub 2009 Oct 29. J Chem Ecol. 2009. PMID: 19866237 Review.
Cited by
-
Desiccation resistance differences in Drosophila species can be largely explained by variations in cuticular hydrocarbons.Elife. 2022 Dec 6;11:e80859. doi: 10.7554/eLife.80859. Elife. 2022. PMID: 36473178 Free PMC article.
-
Genetic Underpinnings of Cuticular Hydrocarbon Biosynthesis in the German Cockroach, Blattella germanica (L.): Progress and Perspectives.J Chem Ecol. 2024 Dec;50(12):955-968. doi: 10.1007/s10886-024-01509-7. Epub 2024 May 10. J Chem Ecol. 2024. PMID: 38727793 Review.
-
The physiology of forager hydration and variation among harvester ant (Pogonomyrmex barbatus) colonies in collective foraging behavior.Sci Rep. 2019 Mar 26;9(1):5126. doi: 10.1038/s41598-019-41586-3. Sci Rep. 2019. PMID: 30914705 Free PMC article.
-
Indigenous and introduced Collembola differ in desiccation resistance but not its plasticity in response to temperature.Curr Res Insect Sci. 2022 Dec 9;3:100051. doi: 10.1016/j.cris.2022.100051. eCollection 2023. Curr Res Insect Sci. 2022. PMID: 36591563 Free PMC article.
-
Cuticular hydrocarbon profiles reveal geographic chemotypes in stingless bees (Hymenoptera: Meliponini).Sci Rep. 2024 Jun 24;14(1):14567. doi: 10.1038/s41598-024-65298-5. Sci Rep. 2024. PMID: 38914659 Free PMC article.
References
-
- Hadley NF. 1994. Water relations in terrestrial arthropods. Chapter 3: Cuticular transpiration. San Diego, CA: Academic Press.
-
- Gibbs AG, Rajpurohit S. 2010. Cuticular lipids and water balance. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Blomquist GJ, Bagnères A-G), pp. 100–120. Cambridge, UK: Cambridge University Press.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

