Negative regulation of Smad1 pathway and collagen IV expression by store-operated Ca2+ entry in glomerular mesangial cells
- PMID: 28298362
- PMCID: PMC5495888
- DOI: 10.1152/ajprenal.00642.2016
Negative regulation of Smad1 pathway and collagen IV expression by store-operated Ca2+ entry in glomerular mesangial cells
Abstract
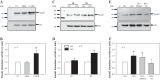
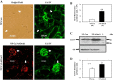
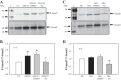
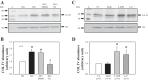
Collagen IV (Col IV) is a major component of expanded glomerular extracellular matrix in diabetic nephropathy and Smad1 is a key molecule regulating Col IV expression in mesangial cells (MCs). The present study was conducted to determine if Smad1 pathway and Col IV protein abundance were regulated by store-operated Ca2+ entry (SOCE). In cultured human MCs, pharmacological inhibition of SOCE significantly increased the total amount of Smad1 protein. Activation of SOCE blunted high-glucose-increased Smad1 protein content. Treatment of human MCs with ANG II at 1 µM for 15 min, high glucose for 3 days, or TGF-β1 at 5 ng/ml for 30 min increased the level of phosphorylated Smad1. However, the phosphorylation of Smad1 by those stimuli was significantly attenuated by activation of SOCE. Knocking down Smad1 reduced, but expressing Smad1 increased, the amount of Col IV protein. Furthermore, activation of SOCE significantly attenuated high-glucose-induced Col IV protein production, and blockade of SOCE substantially increased the abundance of Col IV. To further verify those in vitro findings, we downregulated SOCE specifically in MCs in mice using small-interfering RNA (siRNA) against Orai1 (the channel protein mediating SOCE) delivered by the targeted nanoparticle delivery system. Immunohistochemical examinations showed that expression of both Smad1 and Col IV proteins was significantly greater in the glomeruli with positively transfected Orai1 siRNA compared with the glomeruli from the mice without Orai1 siRNA treatment. Taken together, our results indicate that SOCE negatively regulates the Smad1 signaling pathway and inhibits Col IV protein production in MCs.
Keywords: extracellular matrix; store-operated calcium entry.
Copyright © 2017 the American Physiological Society.
Figures



Similar articles
-
Store-operated calcium entry suppressed the TGF-β1/Smad3 signaling pathway in glomerular mesangial cells.Am J Physiol Renal Physiol. 2017 Sep 1;313(3):F729-F739. doi: 10.1152/ajprenal.00483.2016. Epub 2017 Jun 21. Am J Physiol Renal Physiol. 2017. PMID: 28637791 Free PMC article.
-
High glucose and diabetes enhanced store-operated Ca(2+) entry and increased expression of its signaling proteins in mesangial cells.Am J Physiol Renal Physiol. 2014 May 1;306(9):F1069-80. doi: 10.1152/ajprenal.00463.2013. Epub 2014 Mar 12. Am J Physiol Renal Physiol. 2014. PMID: 24623143 Free PMC article.
-
Inhibition of interleukin-6 on matrix protein production by glomerular mesangial cells and the pathway involved.Am J Physiol Renal Physiol. 2020 Jun 1;318(6):F1478-F1488. doi: 10.1152/ajprenal.00043.2020. Epub 2020 May 11. Am J Physiol Renal Physiol. 2020. PMID: 32390515 Free PMC article.
-
STIM-TRP Pathways and Microdomain Organization: Contribution of TRPC1 in Store-Operated Ca2+ Entry: Impact on Ca2+ Signaling and Cell Function.Adv Exp Med Biol. 2017;993:159-188. doi: 10.1007/978-3-319-57732-6_9. Adv Exp Med Biol. 2017. PMID: 28900914 Review.
-
Current perspectives in the pharmacological studies of store-operated Ca2+ entry blockers.Jpn J Pharmacol. 1999 Nov;81(3):253-8. doi: 10.1254/jjp.81.253. Jpn J Pharmacol. 1999. PMID: 10622212 Review.
Cited by
-
Enhanced Orai1-mediated store-operated Ca2+ channel/calpain signaling contributes to high glucose-induced podocyte injury.J Biol Chem. 2022 Jun;298(6):101990. doi: 10.1016/j.jbc.2022.101990. Epub 2022 Apr 29. J Biol Chem. 2022. PMID: 35490782 Free PMC article.
-
Store-operated calcium entry: Pivotal roles in renal physiology and pathophysiology.Exp Biol Med (Maywood). 2021 Feb;246(3):305-316. doi: 10.1177/1535370220975207. Epub 2020 Nov 29. Exp Biol Med (Maywood). 2021. PMID: 33249888 Free PMC article. Review.
-
Ion channels as a therapeutic target for renal fibrosis.Front Physiol. 2022 Oct 5;13:1019028. doi: 10.3389/fphys.2022.1019028. eCollection 2022. Front Physiol. 2022. PMID: 36277193 Free PMC article. Review.
-
I-mfa, Mesangial Cell TRPC1 Channel, and Regulation of GFR.J Am Soc Nephrol. 2025 Apr 1;36(4):614-627. doi: 10.1681/ASN.0000000533. Epub 2024 Oct 24. J Am Soc Nephrol. 2025. PMID: 39446484
-
Pathophysiology of mesangial expansion in diabetic nephropathy: mesangial structure, glomerular biomechanics, and biochemical signaling and regulation.J Biol Eng. 2022 Aug 2;16(1):19. doi: 10.1186/s13036-022-00299-4. J Biol Eng. 2022. PMID: 35918708 Free PMC article. Review.
References
-
- Abdel-Wahab N, Wicks SJ, Mason RM, Chantry A. Decorin suppresses transforming growth factor-beta-induced expression of plasminogen activator inhibitor-1 in human mesangial cells through a mechanism that involves Ca2+-dependent phosphorylation of Smad2 at serine-240. Biochem J 362: 643–649, 2002. doi:10.1042/bj3620643. - DOI - PMC - PubMed
-
- Anitha M, Shahnavaz N, Qayed E, Joseph I, Gossrau G, Mwangi S, Sitaraman SV, Greene JG, Srinivasan S. BMP2 promotes differentiation of nitrergic and catecholaminergic enteric neurons through a Smad1-dependent pathway. Am J Physiol Gastrointest Liver Physiol 298: G375–G383, 2010. doi:10.1152/ajpgi.00343.2009. - DOI - PMC - PubMed
-
- Araoka T, Abe H, Tominaga T, Mima A, Matsubara T, Murakami T, Kishi S, Nagai K, Doi T. Transcription factor 7-like 2 (TCF7L2) regulates activin receptor-like kinase 1 (ALK1)/Smad1 pathway for development of diabetic nephropathy. Mol Cells 30: 209–218, 2010. doi:10.1007/s10059-010-0109-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

