Mimicry of an HIV broadly neutralizing antibody epitope with a synthetic glycopeptide
- PMID: 28298421
- PMCID: PMC5562351
- DOI: 10.1126/scitranslmed.aai7521
Mimicry of an HIV broadly neutralizing antibody epitope with a synthetic glycopeptide
Abstract
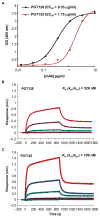
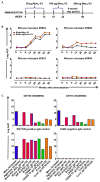
A goal for an HIV-1 vaccine is to overcome virus variability by inducing broadly neutralizing antibodies (bnAbs). One key target of bnAbs is the glycan-polypeptide at the base of the envelope (Env) third variable loop (V3). We have designed and synthesized a homogeneous minimal immunogen with high-mannose glycans reflective of a native Env V3-glycan bnAb epitope (Man9-V3). V3-glycan bnAbs bound to Man9-V3 glycopeptide and native-like gp140 trimers with similar affinities. Fluorophore-labeled Man9-V3 glycopeptides bound to bnAb memory B cells and were able to be used to isolate a V3-glycan bnAb from an HIV-1-infected individual. In rhesus macaques, immunization with Man9-V3 induced V3-glycan-targeted antibodies. Thus, the Man9-V3 glycopeptide closely mimics an HIV-1 V3-glycan bnAb epitope and can be used to isolate V3-glycan bnAbs.
Copyright © 2017, American Association for the Advancement of Science.
Conflict of interest statement
Figures


References
-
- Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, Phogat A, Chakrabarti B, Li Y, Connors M, Pereyra F, Walker BD, Wardemann H, Ho D, Wyatt RT, Mascola JR, Ravetch JV, Nussenzweig MC. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. - PubMed
-
- Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TYK, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. - PMC - PubMed
-
- Wu X, Yang Z-Y, Li Y, Hogerkorp C-M, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O’Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

