SCFFBXO17 E3 ligase modulates inflammation by regulating proteasomal degradation of glycogen synthase kinase-3β in lung epithelia
- PMID: 28298444
- PMCID: PMC5418045
- DOI: 10.1074/jbc.M116.771667
SCFFBXO17 E3 ligase modulates inflammation by regulating proteasomal degradation of glycogen synthase kinase-3β in lung epithelia
Abstract
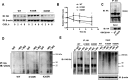
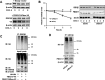
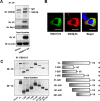
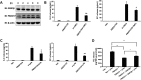
Glycogen synthase kinase-3β (GSK3β) has diverse biological roles including effects on cellular differentiation, migration, and inflammation. GSK3β phosphorylates proteins to generate phosphodegrons necessary for recognition by Skp1/Cullin-1/F-box (SCF) E3 ubiquitin ligases leading to subsequent proteasomal degradation of these substrates. However, little is known regarding how GSK3β protein stability itself is regulated and how its stability may influence inflammation. Here we show that GSK3β is degraded by the ubiquitin-proteasome pathway in murine lung epithelial cells through lysine 183 as an acceptor site for K48 polyubiquitination. We have identified FBXO17 as an F-box protein subunit that recognizes and mediates GSK3β polyubiquitination. Both endogenous and ectopically expressed FBXO17 associate with GSK3β, and its overexpression leads to decreased protein levels of GSK3β. Silencing FBXO17 gene expression increased the half-life of GSK3β in cells. Furthermore, overexpression of FBXO17 inhibits agonist-induced release of keratinocyte-derived cytokine (KC) and interleukin-6 (IL-6) production by cells. Thus, the SCFFBXO17 E3 ubiquitin ligase complex negatively regulates inflammation by targeting GSK3β in lung epithelia.
Keywords: E3 ubiquitin ligase; inflammation; lung; lung injury; ubiquitin.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
Glycogen synthase kinase-3β stabilizes the interleukin (IL)-22 receptor from proteasomal degradation in murine lung epithelia.J Biol Chem. 2014 Jun 20;289(25):17610-9. doi: 10.1074/jbc.M114.551747. Epub 2014 Apr 17. J Biol Chem. 2014. PMID: 24742671 Free PMC article.
-
E3 ubiquitin ligase Fbw7 negatively regulates granulocytic differentiation by targeting G-CSFR for degradation.Biochim Biophys Acta. 2013 Dec;1833(12):2639-2652. doi: 10.1016/j.bbamcr.2013.06.018. Epub 2013 Jun 29. Biochim Biophys Acta. 2013. PMID: 23820376
-
A new mechanism of RhoA ubiquitination and degradation: roles of SCF(FBXL19) E3 ligase and Erk2.Biochim Biophys Acta. 2013 Dec;1833(12):2757-2764. doi: 10.1016/j.bbamcr.2013.07.005. Epub 2013 Jul 18. Biochim Biophys Acta. 2013. PMID: 23871831 Free PMC article.
-
New insights on the function of SCF ubiquitin E3 ligases in the lung.Cell Signal. 2013 Sep;25(9):1792-8. doi: 10.1016/j.cellsig.2013.05.003. Epub 2013 May 14. Cell Signal. 2013. PMID: 23680451 Free PMC article. Review.
-
Proteolytic regulation of metabolic enzymes by E3 ubiquitin ligase complexes: lessons from yeast.Crit Rev Biochem Mol Biol. 2015;50(6):489-502. doi: 10.3109/10409238.2015.1081869. Epub 2015 Sep 11. Crit Rev Biochem Mol Biol. 2015. PMID: 26362128 Review.
Cited by
-
Essential role of IκBNS for in vivo CD4+ T-cell activation, proliferation, and Th1-cell differentiation during Listeria monocytogenes infection in mice.Eur J Immunol. 2019 Sep;49(9):1391-1398. doi: 10.1002/eji.201847961. Epub 2019 Jun 7. Eur J Immunol. 2019. PMID: 31049948 Free PMC article.
-
Crosstalk and Interplay between the Ubiquitin-Proteasome System and Autophagy.Mol Cells. 2017 Jul 31;40(7):441-449. doi: 10.14348/molcells.2017.0115. Epub 2017 Jul 24. Mol Cells. 2017. PMID: 28743182 Free PMC article. Review.
-
HMGB1-Induced p62 Overexpression Promotes Snail-Mediated Epithelial-Mesenchymal Transition in Glioblastoma Cells via the Degradation of GSK-3β.Theranostics. 2019 Mar 16;9(7):1909-1922. doi: 10.7150/thno.30578. eCollection 2019. Theranostics. 2019. PMID: 31037147 Free PMC article.
-
Glycogen synthase kinase 3β: a key player in progressive chronic kidney disease.Clin Sci (Lond). 2025 Jun 17;139(12):605-25. doi: 10.1042/CS20245219. Clin Sci (Lond). 2025. PMID: 40526103 Free PMC article. Review.
-
The Roles of Ubiquitin-Binding Protein Shuttles in the Degradative Fate of Ubiquitinated Proteins in the Ubiquitin-Proteasome System and Autophagy.Cells. 2019 Jan 10;8(1):40. doi: 10.3390/cells8010040. Cells. 2019. PMID: 30634694 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

