The structure and function of Mycobacterium tuberculosis MazF-mt6 toxin provide insights into conserved features of MazF endonucleases
- PMID: 28298445
- PMCID: PMC5427253
- DOI: 10.1074/jbc.M117.779306
The structure and function of Mycobacterium tuberculosis MazF-mt6 toxin provide insights into conserved features of MazF endonucleases
Abstract
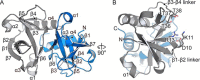
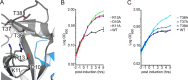
Toxin-antitoxin systems are ubiquitous in prokaryotic and archaeal genomes and regulate growth in response to stress. Escherichia coli contains at least 36 putative toxin-antitoxin gene pairs, and some pathogens such as Mycobacterium tuberculosis have over 90 toxin-antitoxin operons. E. coli MazF cleaves free mRNA after encountering stress, and nine M. tuberculosis MazF family members cleave mRNA, tRNA, or rRNA. Moreover, M. tuberculosis MazF-mt6 cleaves 23S rRNA Helix 70 to inhibit protein synthesis. The overall tertiary folds of these MazFs are predicted to be similar, and therefore, it is unclear how they recognize structurally distinct RNAs. Here we report the 2.7-Å X-ray crystal structure of MazF-mt6. MazF-mt6 adopts a PemK-like fold but lacks an elongated β1-β2 linker, a region that typically acts as a gate to direct RNA or antitoxin binding. In the absence of an elongated β1-β2 linker, MazF-mt6 is unable to transition between open and closed states, suggesting that the regulation of RNA or antitoxin selection may be distinct from other canonical MazFs. Additionally, a shortened β1-β2 linker allows for the formation of a deep, solvent-accessible, active-site pocket, which may allow recognition of specific, structured RNAs like Helix 70. Structure-based mutagenesis and bacterial growth assays demonstrate that MazF-mt6 residues Asp-10, Arg-13, and Thr-36 are critical for RNase activity and likely catalyze the proton-relay mechanism for RNA cleavage. These results provide further critical insights into how MazF secondary structural elements adapt to recognize diverse RNA substrates.
Keywords: X-ray crystallography; bacterial toxin; endoribonuclease; protein synthesis; ribosomal ribonucleic acid (rRNA) (ribosomal RNA); ribosome.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




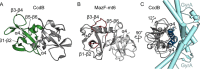
References
-
- Maisonneuve E., Castro-Camargo M., and Gerdes K. (2013) (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell 154, 1140–1150 - PubMed
-
- Yamaguchi Y., Park J. H., and Inouye M. (2011) Toxin-antitoxin systems in bacteria and archaea. Annu. Rev. Genet. 45, 61–79 - PubMed
-
- Lobato-Márquez D., Díaz-Orejas R., and García-Del Portillo F. (2016) Toxin-antitoxins and bacterial virulence. FEMS Microbiol. Rev. 40, 592–609 - PubMed
-
- Gerdes K., and Maisonneuve E. (2012) Bacterial persistence and toxin-antitoxin loci. Annu. Rev. Microbiol. 66, 103–123 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

