Folding of a single domain protein entering the endoplasmic reticulum precedes disulfide formation
- PMID: 28298446
- PMCID: PMC5409466
- DOI: 10.1074/jbc.M117.780742
Folding of a single domain protein entering the endoplasmic reticulum precedes disulfide formation
Abstract
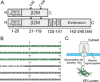
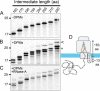
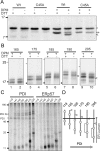
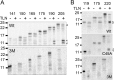
The relationship between protein synthesis, folding, and disulfide formation within the endoplasmic reticulum (ER) is poorly understood. Previous studies have suggested that pre-existing disulfide links are absolutely required to allow protein folding and, conversely, that protein folding occurs prior to disulfide formation. To address the question of what happens first within the ER, that is, protein folding or disulfide formation, we studied folding events at the early stages of polypeptide chain translocation into the mammalian ER using stalled translation intermediates. Our results demonstrate that polypeptide folding can occur without complete domain translocation. Protein disulfide isomerase (PDI) interacts with these early intermediates, but disulfide formation does not occur unless the entire sequence of the protein domain is translocated. This is the first evidence that folding of the polypeptide chain precedes disulfide formation within a cellular context and highlights key differences between protein folding in the ER and refolding of purified proteins.
Keywords: ER; disulfide; disulfide formation; endoplasmic reticulum; protein disulfide isomerase; protein folding; protein translocation; β2-microglobulin.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflict of interest
Figures

References
-
- Fass D. (2012) Disulfide bonding in protein biophysics. Annu. Rev. Biophys. 41, 63–79 - PubMed
-
- Camacho C. J., and Thirumalai D. (1995) Modeling the role of disulfide bonds in protein folding: entropic barriers and pathways. Proteins 22, 27–40 - PubMed
-
- Wallis A. K., and Freedman R. B. (2013) Assisting oxidative protein folding: how do protein disulphide-isomerases couple conformational and chemical processes in protein folding? Top. Curr. Chem. 328, 1–34 - PubMed
-
- Zhang G., and Ignatova Z. (2011) Folding at the birth of the nascent chain: coordinating translation with co-translational folding. Curr. Opin. Struct. Biol. 21, 25–31 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

