Evaluation of Altona Diagnostics RealStar Zika Virus Reverse Transcription-PCR Test Kit for Zika Virus PCR Testing
- PMID: 28298448
- PMCID: PMC5405276
- DOI: 10.1128/JCM.02153-16
Evaluation of Altona Diagnostics RealStar Zika Virus Reverse Transcription-PCR Test Kit for Zika Virus PCR Testing
Abstract
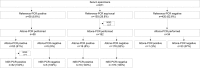
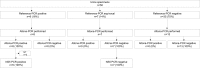
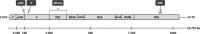
With the emerging Zika virus (ZIKV) epidemic, accessible real-time reverse transcription-PCR (rRT-PCR) assays are needed to streamline testing. The commercial Altona Diagnostics RealStar ZIKV rRT-PCR test kit (Altona PCR) has been approved for emergency use authorization by the U.S. FDA. Our aim was to verify the Altona PCR by comparing it to the CDC-designed dual-target ZIKV rRT-PCR reference assay (reference PCR) and describe the demographics of patients tested for ZIKV by rRT-PCR in Ontario, Canada. A large set of clinical specimens was tested for ZIKV by the Altona PCR and the reference PCR. Positive or equivocal specimens underwent PCR and Sanger sequencing targeting the ZIKV NS5 gene. A total of 671 serum specimens were tested by the reference PCR: 58 (8.6%) were positive, 193 (28.8%) were equivocal, and 420 (62.6%) were negative. Ninety percent of the reference PCR-positive patients were tested in the first 5 days after symptom onset. The Altona PCR was performed on 284/671 specimens tested by the reference PCR. The Altona PCR was positive for 53/58 (91%) reference PCR-positive specimens and 16/193 (8%) reference PCR-equivocal specimens; the ZIKV NS5 PCR was positive for all 68 Altona PCR-positive specimens and negative for all 181 Altona PCR-negative specimens that underwent the NS5 PCR. The Altona PCR has very good sensitivity (91%) and specificity (97%) compared to the reference PCR. The Altona PCR can be used for ZIKV diagnostic testing and has less extensive verification requirements than a laboratory-developed test.
Keywords: PCR; Zika virus; diagnostic testing; flavivirus.
Copyright © 2017 American Society for Microbiology.
Figures

Similar articles
-
Improved detection of Zika virus RNA in human and animal specimens by a novel, highly sensitive and specific real-time RT-PCR assay targeting the 5'-untranslated region of Zika virus.Trop Med Int Health. 2017 May;22(5):594-603. doi: 10.1111/tmi.12857. Epub 2017 Mar 16. Trop Med Int Health. 2017. PMID: 28214373
-
Evaluation of Aptima Zika Virus Assay.J Clin Microbiol. 2017 Jul;55(7):2198-2203. doi: 10.1128/JCM.00603-17. Epub 2017 May 3. J Clin Microbiol. 2017. PMID: 28468854 Free PMC article.
-
ReEBOV Antigen Rapid Test kit for point-of-care and laboratory-based testing for Ebola virus disease: a field validation study.Lancet. 2015 Aug 29;386(9996):867-74. doi: 10.1016/S0140-6736(15)61042-X. Epub 2015 Jun 25. Lancet. 2015. PMID: 26119838
-
Laboratory Diagnosis of Zika Virus Infection.Arch Pathol Lab Med. 2017 Jan;141(1):60-67. doi: 10.5858/arpa.2016-0406-SA. Epub 2016 Oct 20. Arch Pathol Lab Med. 2017. PMID: 27763787 Review.
-
Advantage of urine based molecular diagnosis of Zika virus.Int Urol Nephrol. 2016 Dec;48(12):1961-1966. doi: 10.1007/s11255-016-1406-9. Epub 2016 Aug 27. Int Urol Nephrol. 2016. PMID: 27567913 Review.
Cited by
-
Performance of the Trioplex real-time RT-PCR assay for detection of Zika, dengue, and chikungunya viruses.Nat Commun. 2018 Apr 11;9(1):1391. doi: 10.1038/s41467-018-03772-1. Nat Commun. 2018. PMID: 29643334 Free PMC article.
-
Detection of Anti-ZIKV NS1 IgA, IgM, and Combined IgA/IgM and Identification of IL-4 and IL-10 as Potential Biomarkers for Early ZIKV and DENV Infections in Hyperendemic Regions, Thailand.Trop Med Infect Dis. 2023 May 17;8(5):284. doi: 10.3390/tropicalmed8050284. Trop Med Infect Dis. 2023. PMID: 37235332 Free PMC article.
-
Evaluation of Euroimmun Anti-Zika Virus IgM and IgG Enzyme-Linked Immunosorbent Assays for Zika Virus Serologic Testing.J Clin Microbiol. 2017 Aug;55(8):2462-2471. doi: 10.1128/JCM.00442-17. Epub 2017 May 31. J Clin Microbiol. 2017. PMID: 28566316 Free PMC article.
-
Assessment of a multiplex arbovirus PCR Detection Test in an area endemic for Chikungunya, Zika, and Dengue viruses: An evaluation of kit performance characteristics in line with Clinical Laboratory Improvement Amendments (CLIA) Standards.PLoS One. 2025 Jun 24;20(6):e0309626. doi: 10.1371/journal.pone.0309626. eCollection 2025. PLoS One. 2025. PMID: 40554518 Free PMC article.
-
Comparative Evaluation of Indirect Immunofluorescence and NS-1-Based ELISA to Determine Zika Virus-Specific IgM.Viruses. 2018 Jul 19;10(7):379. doi: 10.3390/v10070379. Viruses. 2018. PMID: 30029548 Free PMC article.
References
-
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. 2009. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 360:2536–2543. doi:10.1056/NEJMoa0805715. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

