Distinct Regulatory Effects of Myeloid Cell and Endothelial Cell NAPDH Oxidase 2 on Blood Pressure
- PMID: 28298457
- PMCID: PMC5444427
- DOI: 10.1161/CIRCULATIONAHA.116.023877
Distinct Regulatory Effects of Myeloid Cell and Endothelial Cell NAPDH Oxidase 2 on Blood Pressure
Abstract
Background: Hypertension caused by increased renin-angiotensin system activation is associated with elevated reactive oxygen species production. Previous studies implicate NADPH oxidase (Nox) proteins as important reactive oxygen species sources during renin-angiotensin system activation, with different Nox isoforms being potentially involved. Among these, Nox2 is expressed in multiple cell types, including endothelial cells, fibroblasts, immune cells, and microglia. Blood pressure (BP) is regulated at the central nervous system, renal, and vascular levels, but the cell-specific role of Nox2 in BP regulation is unknown.
Methods: We generated a novel mouse model with a floxed Nox2 gene and used Tie2-Cre, LysM Cre, or Cdh5-CreERT2 driver lines to develop cell-specific models of Nox2 perturbation to investigate its role in BP regulation.
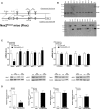
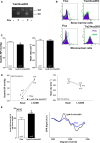
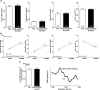
Results: Unexpectedly, Nox2 deletion in myeloid but not endothelial cells resulted in a significant reduction in basal BP. Both Tie2-CreNox2 knockout (KO) mice (in which Nox2 was deficient in both endothelial cells and myeloid cells) and LysM CreNox2KO mice (in which Nox2 was deficient in myeloid cells) had significantly lower BP than littermate controls, whereas basal BP was unaltered in Cdh5-CreERT2 Nox2KO mice (in which Nox2 is deficient only in endothelial cells). The lower BP was attributable to an increased NO bioavailability that dynamically dilated resistance vessels in vivo under basal conditions without a change in renal function. Myeloid-specific Nox2 deletion had no effect on angiotensin II-induced hypertension, which, however, was blunted in Tie2-CreNox2KO mice, along with preservation of endothelium-dependent relaxation during angiotensin II stimulation.
Conclusions: We identify a hitherto unrecognized modulation of basal BP by myeloid cell Nox2, whereas endothelial cell Nox2 regulates angiotensin II-induced hypertension. These results identify distinct cell-specific roles for Nox2 in BP regulation.
Keywords: NADPH oxidase; angiotensin II; blood pressure; mice.
© 2017 The Authors.
Figures




Comment in
-
Letter by Violi et al Regarding Article, "Distinct Regulatory Effects of Myeloid Cell and Endothelial Cell NAPDH Oxidase 2 on Blood Pressure".Circulation. 2017 Nov 21;136(21):2088-2089. doi: 10.1161/CIRCULATIONAHA.117.028607. Circulation. 2017. PMID: 29158218 No abstract available.
-
Response by Sag et al to Letter Regarding Article, "Distinct Regulatory Effects of Myeloid Cell and Endothelial Cell NAPDH Oxidase 2 on Blood Pressure".Circulation. 2017 Nov 21;136(21):2090-2091. doi: 10.1161/CIRCULATIONAHA.117.030515. Circulation. 2017. PMID: 29158219 No abstract available.
References
-
- Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. - PubMed
-
- Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med. 2002;346:1954–1962. doi: 10.1056/NEJMoa013591. - PubMed
-
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. - PubMed
-
- Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res. 2012;111:1091–1106. doi: 10.1161/CIRCRESAHA.111.255216. - PubMed
-
- Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR HOPE and HOPE-TOO Trial Investigators. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

