BK Polyomavirus: Clinical Aspects, Immune Regulation, and Emerging Therapies
- PMID: 28298471
- PMCID: PMC5355639
- DOI: 10.1128/CMR.00074-16
BK Polyomavirus: Clinical Aspects, Immune Regulation, and Emerging Therapies
Abstract
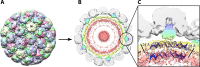
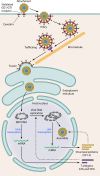
BK polyomavirus (BKV) causes frequent infections during childhood and establishes persistent infections within renal tubular cells and the uroepithelium, with minimal clinical implications. However, reactivation of BKV in immunocompromised individuals following renal or hematopoietic stem cell transplantation may cause serious complications, including BKV-associated nephropathy (BKVAN), ureteric stenosis, or hemorrhagic cystitis. Implementation of more potent immunosuppression and increased posttransplant surveillance has resulted in a higher incidence of BKVAN. Antiviral immunity plays a crucial role in controlling BKV replication, and our increasing knowledge about host-virus interactions has led to the development of improved diagnostic tools and clinical management strategies. Currently, there are no effective antiviral agents for BKV infection, and the mainstay of managing reactivation is reduction of immunosuppression. Development of immune-based therapies to combat BKV may provide new and exciting opportunities for the successful treatment of BKV-associated complications.
Keywords: B cell responses; T cell immunity; T cells; immunotherapy; natural killer cells; pathogenesis; transplant; virus.
Copyright © 2017 American Society for Microbiology.
Figures







References
-
- Gardner SD, Field AM, Coleman DV, Hulme B. 1971. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet i:1253–1257. - PubMed
-
- Chatterjee M, Weyandt TB, Frisque RJ. 2000. Identification of archetype and rearranged forms of BK virus in leukocytes from healthy individuals. J Med Virol 60:353–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

