A Single-Dose Recombinant Parainfluenza Virus 5-Vectored Vaccine Expressing Respiratory Syncytial Virus (RSV) F or G Protein Protected Cotton Rats and African Green Monkeys from RSV Challenge
- PMID: 28298602
- PMCID: PMC5432884
- DOI: 10.1128/JVI.00066-17
A Single-Dose Recombinant Parainfluenza Virus 5-Vectored Vaccine Expressing Respiratory Syncytial Virus (RSV) F or G Protein Protected Cotton Rats and African Green Monkeys from RSV Challenge
Abstract
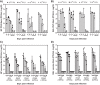
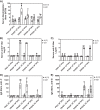
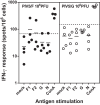
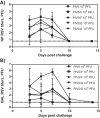
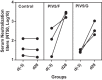
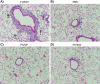
Human respiratory syncytial virus (RSV) is a common cause of severe respiratory disease among infants, immunocompromised individuals, and the elderly. No licensed vaccine is currently available. In this study, we evaluated two parainfluenza virus 5 (PIV5)-vectored vaccines expressing RSV F (PIV5/F) or G (PIV5/G) protein in the cotton rat and African green monkey models for their replication, immunogenicity, and efficacy of protection against RSV challenge. Following a single intranasal inoculation, both animal species shed the vaccine viruses for a limited time but without noticeable clinical symptoms. In cotton rats, the vaccines elicited RSV F- or G-specific serum antibodies and conferred complete lung protection against RSV challenge at doses as low as 103 PFU. Neither vaccine produced the enhanced lung pathology observed in animals immunized with formalin-inactivated RSV. In African green monkeys, vaccine-induced serum and mucosal antibody responses were readily detected, as well. PIV5/F provided nearly complete protection against RSV infection in the upper and lower respiratory tract at a dose of 106 PFU of vaccine. At the same dose levels, PIV5/G was less efficacious. Both PIV5/F and PIV5/G were also able to boost neutralization titers in RSV-preexposed African green monkeys. Overall, our data indicated that PIV5/F is a promising RSV vaccine candidate.IMPORTANCE A safe and efficacious respiratory syncytial virus (RSV) vaccine remains elusive. We tested the recombinant parainfluenza virus 5 (PIV5) vectors expressing RSV glycoproteins for their immunogenicity and protective efficacy in cotton rats and African green monkeys, which are among the best available animal models to study RSV infection. In both species, a single dose of intranasal immunization with PIV5-vectored vaccines was able to produce systemic and local immunity and to protect animals from RSV challenge. The vaccines could also boost RSV neutralization antibody titers in African green monkeys that had been infected previously. Our data suggest that PIV5-vectored vaccines could potentially protect both the pediatric and elderly populations and support continued development of the vector platform.
Keywords: PIV5 vectors; respiratory syncytial virus; vaccines.
Copyright © 2017 American Society for Microbiology.
Figures
Similar articles
-
Mucosal Delivery of Recombinant Vesicular Stomatitis Virus Vectors Expressing Envelope Proteins of Respiratory Syncytial Virus Induces Protective Immunity in Cotton Rats.J Virol. 2021 Feb 24;95(6):e02345-20. doi: 10.1128/JVI.02345-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33408176 Free PMC article.
-
Parainfluenza Virus 5 Expressing Wild-Type or Prefusion Respiratory Syncytial Virus (RSV) Fusion Protein Protects Mice and Cotton Rats from RSV Challenge.J Virol. 2017 Sep 12;91(19):e00560-17. doi: 10.1128/JVI.00560-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28747496 Free PMC article.
-
Genetic Stability of Parainfluenza Virus 5-Vectored Human Respiratory Syncytial Virus Vaccine Candidates after In Vitro and In Vivo Passage.J Virol. 2017 Sep 12;91(19):e00559-17. doi: 10.1128/JVI.00559-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28747497 Free PMC article.
-
Recombinant Sendai virus as a novel vaccine candidate for respiratory syncytial virus.Viral Immunol. 2005;18(2):255-66. doi: 10.1089/vim.2005.18.255. Viral Immunol. 2005. PMID: 16035938 Review.
-
Sendai Virus-Vectored Vaccines That Express Envelope Glycoproteins of Respiratory Viruses.Viruses. 2021 May 29;13(6):1023. doi: 10.3390/v13061023. Viruses. 2021. PMID: 34072332 Free PMC article. Review.
Cited by
-
Intranasal parainfluenza virus type 5 (PIV5)-vectored RSV vaccine is safe and immunogenic in healthy adults in a phase 1 clinical study.Sci Adv. 2023 Oct 27;9(43):eadj7611. doi: 10.1126/sciadv.adj7611. Epub 2023 Oct 25. Sci Adv. 2023. PMID: 37878713 Free PMC article. Clinical Trial.
-
Mucosal Delivery of Recombinant Vesicular Stomatitis Virus Vectors Expressing Envelope Proteins of Respiratory Syncytial Virus Induces Protective Immunity in Cotton Rats.J Virol. 2021 Feb 24;95(6):e02345-20. doi: 10.1128/JVI.02345-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33408176 Free PMC article.
-
A chimeric influenza hemagglutinin delivered by parainfluenza virus 5 vector induces broadly protective immunity against genetically divergent influenza a H1 viruses in swine.Vet Microbiol. 2020 Nov;250:108859. doi: 10.1016/j.vetmic.2020.108859. Epub 2020 Sep 18. Vet Microbiol. 2020. PMID: 33039727 Free PMC article.
-
Combined intranasal and intramuscular parainfluenza 5-, simian adenovirus ChAdOx1- and poxvirus MVA-vectored vaccines induce synergistically HIV-1-specific T cells in the mucosa.Front Immunol. 2023 Jul 17;14:1186478. doi: 10.3389/fimmu.2023.1186478. eCollection 2023. Front Immunol. 2023. PMID: 37529048 Free PMC article.
-
Parainfluenza virus 5-vectored vaccines against human and animal infectious diseases.Rev Med Virol. 2018 Mar;28(2):e1965. doi: 10.1002/rmv.1965. Epub 2018 Jan 5. Rev Med Virol. 2018. PMID: 29316047 Free PMC article. Review.
References
-
- Collins PL, Karen RA. 2013. Respiratory syncytial virus and metapneumovirus, p 1086 In Knipe DM, Howley PM (ed), Fields virology, 6th ed Lippincott, Williams and Wilkins, Philadelphia, PA.
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi:10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

