The impact of temperature changes on vector-borne disease transmission: Culicoides midges and bluetongue virus
- PMID: 28298609
- PMCID: PMC5378124
- DOI: 10.1098/rsif.2016.0481
The impact of temperature changes on vector-borne disease transmission: Culicoides midges and bluetongue virus
Abstract
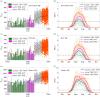
It is a long recognized fact that climatic variations, especially temperature, affect the life history of biting insects. This is particularly important when considering vector-borne diseases, especially in temperate regions where climatic fluctuations are large. In general, it has been found that most biological processes occur at a faster rate at higher temperatures, although not all processes change in the same manner. This differential response to temperature, often considered as a trade-off between onward transmission and vector life expectancy, leads to the total transmission potential of an infected vector being maximized at intermediate temperatures. Here we go beyond the concept of a static optimal temperature, and mathematically model how realistic temperature variation impacts transmission dynamics. We use bluetongue virus (BTV), under UK temperatures and transmitted by Culicoides midges, as a well-studied example where temperature fluctuations play a major role. We first consider an optimal temperature profile that maximizes transmission, and show that this is characterized by a warm day to maximize biting followed by cooler weather to maximize vector life expectancy. This understanding can then be related to recorded representative temperature patterns for England, the UK region which has experienced BTV cases, allowing us to infer historical transmissibility of BTV, as well as using forecasts of climate change to predict future transmissibility. Our results show that when BTV first invaded northern Europe in 2006 the cumulative transmission intensity was higher than any point in the last 50 years, although with climate change such high risks are the expected norm by 2050. Such predictions would indicate that regular BTV epizootics should be expected in the UK in the future.
Keywords: European epizootic outbreaks; climate change; vector-borne disease in northern Palaearctic.
© 2017 The Author(s).
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

