Combined transplantation of human mesenchymal stem cells and human retinal progenitor cells into the subretinal space of RCS rats
- PMID: 28298640
- PMCID: PMC5428026
- DOI: 10.1038/s41598-017-00241-5
Combined transplantation of human mesenchymal stem cells and human retinal progenitor cells into the subretinal space of RCS rats
Abstract
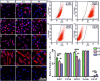
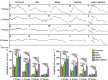
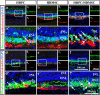
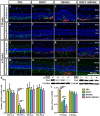
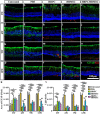
Retinitis pigmentosa (RP) is one of hereditary retinal diseases characterized by the loss of photoreceptors. Cell transplantation has been clinically applied to treat RP patients. Human retinal progenitor cells (HRPCs) and human bone marrow-derived mesenchymal stem cells (HBMSCs) are the two commonly and practically used stem cells for transplantation. Since combined transplantation could be a promising way to integrate the advantages of both stem cell types, we transplanted HRPCs and HBMSCs into the subretinal space (SRS) of Royal College of Surgeons (RCS) rats. We report that HRPCs/HBMSCs combined transplantation maintains the electroretinogram results much better than HRPCs or HBMSCs single transplantations. The thickness of outer nuclear layer also presented a better outcome in the combined transplantation. Importantly, grafted cells in the combination migrated better, both longitudinally and latitudinally, than single transplantation. The photoreceptor differentiation of grafted cells in the retina of RCS rats receiving combined transplantation also showed a higher ratio than single transplantation. Finally, activation of microglia and the gliosis of Müller cells were more effectively suppressed in combined transplantation, indicating better immunomodulatory and anti-gliosis effects. Taken together, combining the transplantation of HRPCs and HBMSCs is a more effective strategy in stem cell-based therapy for retinal degenerative diseases.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

