MKL1 defines the H3K4Me3 landscape for NF-κB dependent inflammatory response
- PMID: 28298643
- PMCID: PMC5428227
- DOI: 10.1038/s41598-017-00301-w
MKL1 defines the H3K4Me3 landscape for NF-κB dependent inflammatory response
Abstract
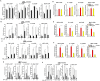
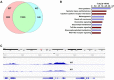
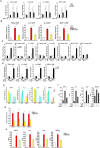
Macrophage-dependent inflammatory response is considered a pivotal biological process that contributes to a host of diseases when aberrantly activated. The underlying epigenetic mechanism is not completely understood. We report here that MKL1 was both sufficient and necessary for p65-dependent pro-inflammatory transcriptional program in immortalized macrophages, in primary human and mouse macrophages, and in an animal model of systemic inflammation (endotoxic shock). Extensive chromatin immunoprecipitation (ChIP) profiling and ChIP-seq analyses revealed that MKL1 deficiency erased key histone modifications synonymous with transactivation on p65 target promoters. Specifically, MKL1 defined histone H3K4 trimethylation landscape for NF-κB dependent transcription. MKL1 recruited an H3K4 trimethyltransferase SET1 to the promoter regions of p65 target genes. There, our work has identified a novel modifier of p65-dependent pro-inflammatory transcription, which may serve as potential therapeutic targets in treating inflammation related diseases.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Natoli G. Specialized chromatin patterns in the control of inflammatory gene expression. Current topics in microbiology and immunology. 2011;349:61–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

