Mapping and Analysis of the Connectome of Sympathetic Premotor Neurons in the Rostral Ventrolateral Medulla of the Rat Using a Volumetric Brain Atlas
- PMID: 28298886
- PMCID: PMC5331070
- DOI: 10.3389/fncir.2017.00009
Mapping and Analysis of the Connectome of Sympathetic Premotor Neurons in the Rostral Ventrolateral Medulla of the Rat Using a Volumetric Brain Atlas
Abstract
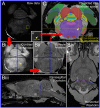
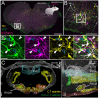
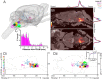
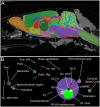
Spinally projecting neurons in the rostral ventrolateral medulla (RVLM) play a critical role in the generation of vasomotor sympathetic tone and are thought to receive convergent input from neurons at every level of the neuraxis; the factors that determine their ongoing activity remain unresolved. In this study we use a genetically restricted viral tracing strategy to definitively map their spatially diffuse connectome. We infected bulbospinal RVLM neurons with a recombinant rabies variant that drives reporter expression in monosynaptically connected input neurons and mapped their distribution using an MRI-based volumetric atlas and a novel image alignment and visualization tool that efficiently translates the positions of neurons captured in conventional photomicrographs to Cartesian coordinates. We identified prominent inputs from well-established neurohumoral and viscero-sympathetic sensory actuators, medullary autonomic and respiratory subnuclei, and supramedullary autonomic nuclei. The majority of inputs lay within the brainstem (88-94%), and included putative respiratory neurons in the pre-Bötzinger Complex and post-inspiratory complex that are therefore likely to underlie respiratory-sympathetic coupling. We also discovered a substantial and previously unrecognized input from the region immediately ventral to nucleus prepositus hypoglossi. In contrast, RVLM sympathetic premotor neurons were only sparsely innervated by suprapontine structures including the paraventricular nucleus, lateral hypothalamus, periaqueductal gray, and superior colliculus, and we found almost no evidence of direct inputs from the cortex or amygdala. Our approach can be used to quantify, standardize and share complete neuroanatomical datasets, and therefore provides researchers with a platform for presentation, analysis and independent reanalysis of connectomic data.
Keywords: RVLM; connectome; mesoscale; rabies; respiratory-sympathetic; segmentation; sympathetic; volumetric.
Figures


References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

