Two Phase Modulation of [Formula: see text] Entry and Cl-/[Formula: see text] Exchanger in Submandibular Glands Cells by Dexmedetomidine
- PMID: 28298895
- PMCID: PMC5331071
- DOI: 10.3389/fphys.2017.00086
Two Phase Modulation of [Formula: see text] Entry and Cl-/[Formula: see text] Exchanger in Submandibular Glands Cells by Dexmedetomidine
Abstract
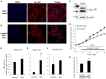
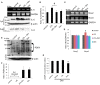
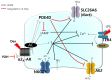
Dexmedetomidine (Dex), a highly selective α2-adrenoceptor agonist, attenuates inflammatory responses induced by lipopolysaccharide (LPS) and induces sedative and analgesic effects. Administration of Dex also reduces salivary secretion in human subjects and inhibits osmotic water permeability in rat cortical collecting ducts. However, little is known about the mechanisms underlying the effects of Dex on salivary glands fluid secretion. We demonstrated the α2-adrenoceptor expression in the basolateral membrane of mouse submandibular glands (SMG). To investigate fluid secretion upon treatment with Dex, we studied the effects of Dex on the activity of Na+-K+-2Cl- cotransporter1 (NKCC1) and Cl-/[Formula: see text] exchange (CBE), and on downstream pro-inflammatory cytokine expression in isolated primary mouse SMG cells. Dex acutely increased CBE activity and NKCC1-mediated and independent [Formula: see text] entry in SMG duct cells, and enhanced ductal fluid secretion in a sealed duct system. Dex showed differential effects on cholinergic/adrenergic stimulations and inflammatory mediators, histamine, and LPS, stimulations-induced Ca2+ in mouse SMG cells. Both, histamine- and LPS-induced intracellular Ca2+ increases were inhibited by Dex, whereas carbachol-stimulated Ca2+ signals were not. Long-lasting (2 h) treatment with Dex reduced CBE activity in SMG and in human submandibular glands (HSG) cells. Moreover, when isolated SMG cells were stimulated with Dex for 2 h, phosphodiesterase 4D (PDE4D) expression was enhanced. These results confirm the anti-inflammatory properties of Dex on LPS-mediated signaling. Further, Dex also inhibited mRNA expression of interleukin-6 and NADPH oxidase 4. The present study also showed that α2-adrenoceptor activation by Dex reduces salivary glands fluid secretion by increasing PDE4D expression, and subsequently reducing the concentration of cAMP. These findings reveal an interaction between the α2-adrenoceptor and PDE4D, which should be considered when using α2-adrenoceptor agonists as sedative or analgesics.
Keywords: dexmedetomidine; ion transporters; phosphodiesterase 4; secretion; submandibular gland.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

