Cathepsin E Deficiency Ameliorates Graft-versus-Host Disease and Modifies Dendritic Cell Motility
- PMID: 28298913
- PMCID: PMC5331043
- DOI: 10.3389/fimmu.2017.00203
Cathepsin E Deficiency Ameliorates Graft-versus-Host Disease and Modifies Dendritic Cell Motility
Abstract
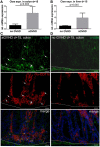
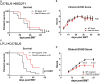
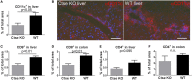
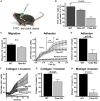
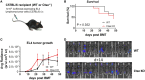
Microbial products influence immunity after allogeneic hematopoietic stem cell transplantation (allo-SCT). In this context, the role of cathepsin E (Ctse), an aspartate protease known to cleave bacterial peptides for antigen presentation in dendritic cells (DCs), has not been studied. During experimental acute graft-versus-host disease (GVHD), we found infiltration by Ctse-positive immune cells leading to higher Ctse RNA- and protein levels in target organs. In Ctse-deficient allo-SCT recipients, we found ameliorated GVHD, improved survival, and lower numbers of tissue-infiltrating DCs. Donor T cell proliferation was not different in Ctse-deficient vs. wild-type allo-SCT recipients in MHC-matched and MHC-mismatched models. Furthermore, Ctse-deficient DCs had an intact ability to induce allogeneic T cell proliferation, suggesting that its role in antigen presentation may not be the main mechanism how Ctse impacts GVHD. We found that Ctse deficiency significantly decreases DC motility in vivo, reduces adhesion to extracellular matrix (ECM), and diminishes invasion through ECM. We conclude that Ctse has a previously unrecognized role in regulating DC motility that possibly contributes to reduced DC counts and ameliorated inflammation in GVHD target organs of Ctse-deficient allo-SCT recipients. However, our data do not provide definite proof that the observed effect of Ctse-/- deficiency is exclusively mediated by DCs. A contribution of Ctse-/--mediated functions in other recipient cell types, e.g., macrophages, cannot be excluded.
Keywords: GVHD; HSCT; cathepsin E; dendritic cells; motility.
Figures


Similar articles
-
Allergic airway inflammation in mice deficient for the antigen-processing protease cathepsin E.Int Arch Allergy Immunol. 2012;159(4):367-83. doi: 10.1159/000338288. Epub 2012 Jul 26. Int Arch Allergy Immunol. 2012. PMID: 22846634
-
Hematopoietic stem cell graft manipulation as a mechanism of immunotherapy.Int Immunopharmacol. 2003 Aug;3(8):1121-43. doi: 10.1016/S1567-5769(03)00014-6. Int Immunopharmacol. 2003. PMID: 12860168 Review.
-
Reduced intensity conditioning for allogeneic hematopoietic stem-cell transplant determines the kinetics of acute graft-versus-host disease.Transplantation. 2008 Oct 15;86(7):968-76. doi: 10.1097/TP.0b013e3181874787. Transplantation. 2008. PMID: 18852664
-
Host-derived CD8⁺ dendritic cells protect against acute graft-versus-host disease after experimental allogeneic bone marrow transplantation.Biol Blood Marrow Transplant. 2014 Nov;20(11):1696-704. doi: 10.1016/j.bbmt.2014.08.005. Epub 2014 Aug 13. Biol Blood Marrow Transplant. 2014. PMID: 25132527
-
Targeting the IL17 pathway for the prevention of graft-versus-host disease.Biol Blood Marrow Transplant. 2014 Jun;20(6):752-9. doi: 10.1016/j.bbmt.2014.02.007. Epub 2014 Feb 22. Biol Blood Marrow Transplant. 2014. PMID: 24565991 Review.
Cited by
-
Spatio-Temporal Bone Remodeling after Hematopoietic Stem Cell Transplantation.Int J Mol Sci. 2020 Dec 29;22(1):267. doi: 10.3390/ijms22010267. Int J Mol Sci. 2020. PMID: 33383915 Free PMC article.
-
Bioinformatics-based screening of key genes associated with gemcitabine resistance in advanced pancreatic ductal adenocarcinoma.Transl Cancer Res. 2024 Dec 31;13(12):6947-6955. doi: 10.21037/tcr-2024-2374. Epub 2024 Dec 27. Transl Cancer Res. 2024. PMID: 39816538 Free PMC article.
-
Innate Immune Determinants of Graft-Versus-Host Disease and Bidirectional Immune Tolerance in Allogeneic Transplantation.OBM Transplant. 2019;3(1):10.21926/obm.transplant.1901044. doi: 10.21926/obm.transplant.1901044. Epub 2019 Jan 31. OBM Transplant. 2019. PMID: 33511333 Free PMC article.
-
A novel efferocytosis-related gene signature for predicting prognosis and therapeutic response in bladder cancer.Sci Rep. 2025 Jun 6;15(1):19912. doi: 10.1038/s41598-025-04037-w. Sci Rep. 2025. PMID: 40481075 Free PMC article.
-
Generation of a C57BL/6J mouse strain expressing the CD45.1 epitope to improve hematopoietic stem cell engraftment and adoptive cell transfer experiments.Lab Anim (NY). 2023 Dec;52(12):324-331. doi: 10.1038/s41684-023-01275-1. Epub 2023 Nov 28. Lab Anim (NY). 2023. PMID: 38017180
References
-
- Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med (2016) 8:339ra371.10.1126/scitranslmed.aaf2311 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

