Biological Characteristics of Fluorescent Superparamagnetic Iron Oxide Labeled Human Dental Pulp Stem Cells
- PMID: 28298928
- PMCID: PMC5337366
- DOI: 10.1155/2017/4837503
Biological Characteristics of Fluorescent Superparamagnetic Iron Oxide Labeled Human Dental Pulp Stem Cells
Abstract
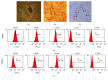
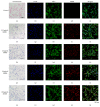
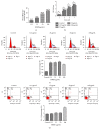
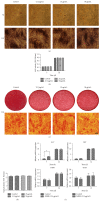
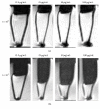
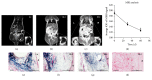
Tracking transplanted stem cells is necessary to clarify cellular properties and improve transplantation success. In this study, we investigate the effects of fluorescent superparamagnetic iron oxide particles (SPIO) (Molday ION Rhodamine-B™, MIRB) on biological properties of human dental pulp stem cells (hDPSCs) and monitor hDPSCs in vitro and in vivo using magnetic resonance imaging (MRI). Morphological analysis showed that intracellular MIRB particles were distributed in the cytoplasm surrounding the nuclei of hDPSCs. 12.5-100 μg/mL MIRB all resulted in 100% labeling efficiency. MTT showed that 12.5-50 μg/mL MIRB could promote cell proliferation and MIRB over 100 μg/mL exhibited toxic effect on hDPSCs. In vitro MRI showed that 1 × 106 cells labeled with various concentrations of MIRB (12.5-100 μg/mL) could be visualized. In vivo MRI showed that transplanted cells could be clearly visualized up to 60 days after transplantation. These results suggest that 12.5-50 μg/mL MIRB is a safe range for labeling hDPSCs. MIRB labeled hDPSCs cell can be visualized by MRI in vitro and in vivo. These data demonstrate that MIRB is a promising candidate for hDPSCs tracking in hDPSCs based dental pulp regeneration therapy.
Conflict of interest statement
The authors have no conflict of interests to declare.
Figures

Similar articles
-
Tracking of Stem Cells from Human Exfoliated Deciduous Teeth Labeled with Molday ION Rhodamine-B during Periodontal Bone Regeneration in Rats.Int J Stem Cells. 2023 Feb 28;16(1):93-107. doi: 10.15283/ijsc21204. Epub 2022 Aug 31. Int J Stem Cells. 2023. PMID: 36042010 Free PMC article.
-
Effects of the iron oxide nanoparticle Molday ION Rhodamine B on the viability and regenerative function of neural stem cells: relevance to clinical translation.Int J Nanomedicine. 2016 Apr 27;11:1731-48. doi: 10.2147/IJN.S102006. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27175074 Free PMC article.
-
Mesenchymal stem cell labeling and in vitro MR characterization at 1.5 T of new SPIO contrast agent: Molday ION Rhodamine-B™.Contrast Media Mol Imaging. 2011 Jan-Feb;6(1):7-18. doi: 10.1002/cmmi.396. Epub 2010 Aug 5. Contrast Media Mol Imaging. 2011. PMID: 20690161 Free PMC article.
-
Optimal technique for canine mesenchymal stem cells labeling with novel SPIO, MIRB™: for MRI detection of transplanted stem cells canine stroke model.Neurol Res. 2024 Apr;46(4):326-329. doi: 10.1080/01616412.2024.2303879. Epub 2024 Mar 11. Neurol Res. 2024. PMID: 38468486
-
Impacts of fluorescent superparamagnetic iron oxide (SPIO)-labeled materials on biological characteristics and osteogenesis of bone marrow mesenchymal stem cells (BMSCs).Int J Clin Exp Med. 2015 Aug 15;8(8):12172-81. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26550127 Free PMC article.
Cited by
-
Tracking of Stem Cells from Human Exfoliated Deciduous Teeth Labeled with Molday ION Rhodamine-B during Periodontal Bone Regeneration in Rats.Int J Stem Cells. 2023 Feb 28;16(1):93-107. doi: 10.15283/ijsc21204. Epub 2022 Aug 31. Int J Stem Cells. 2023. PMID: 36042010 Free PMC article.
-
Enhanced Homing of Mesenchymal Stem Cells Overexpressing Fibroblast Growth Factor 21 to Injury Site in a Mouse Model of Traumatic Brain Injury.Int J Mol Sci. 2019 May 28;20(11):2624. doi: 10.3390/ijms20112624. Int J Mol Sci. 2019. PMID: 31142002 Free PMC article.
-
Triple-modal imaging of stem-cells labeled with multimodal nanoparticles, applied in a stroke model.World J Stem Cells. 2019 Feb 26;11(2):100-123. doi: 10.4252/wjsc.v11.i2.100. World J Stem Cells. 2019. PMID: 30842808 Free PMC article.
-
Superparamagnetic Iron Oxide Nanoparticles: Cytotoxicity, Metabolism, and Cellular Behavior in Biomedicine Applications.Int J Nanomedicine. 2021 Aug 31;16:6097-6113. doi: 10.2147/IJN.S321984. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34511908 Free PMC article. Review.
References
-
- Isobe Y., Koyama N., Nakao K., et al. Comparison of human mesenchymal stem cells derived from bone marrow, synovial fluid, adult dental pulp, and exfoliated deciduous tooth pulp. International Journal of Oral and Maxillofacial Surgery. 2016;45(1):124–131. doi: 10.1016/j.ijom.2015.06.022. - DOI - PubMed
-
- Shen W.-B., Plachez C., Chan A., et al. Human neural progenitor cells retain viability, phenotype, proliferation, and lineage differentiation when labeled with a novel iron oxide nanoparticle, Molday ION Rhodamine B. International Journal of Nanomedicine. 2013;8:4593–4600. doi: 10.2147/IJN.S53012. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

