Cardiomyocyte Ogt limits ventricular dysfunction in mice following pressure overload without affecting hypertrophy
- PMID: 28299467
- PMCID: PMC5555162
- DOI: 10.1007/s00395-017-0612-7
Cardiomyocyte Ogt limits ventricular dysfunction in mice following pressure overload without affecting hypertrophy
Abstract
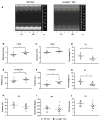
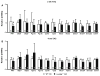
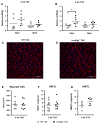
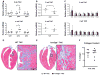
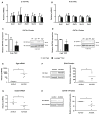
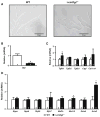
The myocardial response to pressure overload involves coordination of multiple transcriptional, posttranscriptional, and metabolic cues. The previous studies show that one such metabolic cue, O-GlcNAc, is elevated in the pressure-overloaded heart, and the increase in O-GlcNAcylation is required for cardiomyocyte hypertrophy in vitro. Yet, it is not clear whether and how O-GlcNAcylation participates in the hypertrophic response in vivo. Here, we addressed this question using patient samples and a preclinical model of heart failure. Protein O-GlcNAcylation levels were increased in myocardial tissue from heart failure patients compared with normal patients. To test the role of OGT in the heart, we subjected cardiomyocyte-specific, inducibly deficient Ogt (i-cmOgt -/-) mice and Ogt competent littermate wild-type (WT) mice to transverse aortic constriction. Deletion of cardiomyocyte Ogt significantly decreased O-GlcNAcylation and exacerbated ventricular dysfunction, without producing widespread changes in metabolic transcripts. Although some changes in hypertrophic and fibrotic signaling were noted, there were no histological differences in hypertrophy or fibrosis. We next determined whether significant differences were present in i-cmOgt -/- cardiomyocytes from surgically naïve mice. Interestingly, markers of cardiomyocyte dedifferentiation were elevated in Ogt-deficient cardiomyocytes. Although no significant differences in cardiac dysfunction were apparent after recombination, it is possible that such changes in dedifferentiation markers could reflect a larger phenotypic shift within the Ogt-deficient cardiomyocytes. We conclude that cardiomyocyte Ogt is not required for cardiomyocyte hypertrophy in vivo; however, loss of Ogt may exert subtle phenotypic differences in cardiomyocytes that sensitize the heart to pressure overload-induced ventricular dysfunction.
Keywords: Glycosylation; Heart failure; Hexosamine biosynthetic pathway; Metabolism.
Figures


References
-
- Bisping E, Ikeda S, Kong SW, Tarnavski O, Bodyak N, McMullen JR, Rajagopal S, Son JK, Ma Q, Springer Z, Kang PM, Izumo S, Pu WT. Gata4 is required for maintenance of postnatal cardiac function and protection from pressure overload-induced heart failure. Proc Natl Acad Sci USA. 2006;103:14471–14476. doi: 10.1073/pnas.0602543103. - DOI - PMC - PubMed
-
- Condorelli G, Roncarati R, Ross J, Jr, Pisani A, Stassi G, Todaro M, Trocha S, Drusco A, Gu Y, Russo MA, Frati G, Jones SP, Lefer DJ, Napoli C, Croce CM. Heart-targeted overexpression of caspase3 in mice increases infarct size and depresses cardiac function. Proc Natl Acad Sci USA. 2001;98:9977–9982. doi: 10.1073/pnas.161120198. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

