Prognostic and predictive value of circulating tumor cells and CXCR4 expression as biomarkers for a CXCR4 peptide antagonist in combination with carboplatin-etoposide in small cell lung cancer: exploratory analysis of a phase II study
- PMID: 28299514
- PMCID: PMC5418321
- DOI: 10.1007/s10637-017-0446-z
Prognostic and predictive value of circulating tumor cells and CXCR4 expression as biomarkers for a CXCR4 peptide antagonist in combination with carboplatin-etoposide in small cell lung cancer: exploratory analysis of a phase II study
Abstract
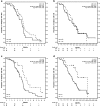
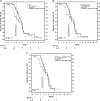
Background Circulating tumor cells (CTCs) and chemokine (C-X-C motif) receptor 4 (CXCR4) expression in CTCs and tumor tissue were evaluated as prognostic or predictive markers of CXCR4 peptide antagonist LY2510924 plus carboplatin-etoposide (CE) versus CE in extensive-stage disease small cell lung cancer (ED-SCLC). Methods This exploratory analysis of a phase II study evaluated CXCR4 expression in baseline tumor tissue and peripheral blood CTCs and in post-treatment CTCs. Optimum cutoff values were determined for CTC counts and CXCR4 expression in tumors and CTCs as predictors of survival outcome. Kaplan-Meier estimates and hazard ratios were used to determine biomarker prognostic and predictive values. Results There was weak positive correlation at baseline between CXCR4 expression in tumor tissue and CTCs. Optimum cutoff values were H-score ≥ 210 for CXCR4+ tumor, ≥7% CTCs with CXCR4 expression (CXCR4+ CTCs), and ≥6 CTCs/7.5 mL blood. Baseline H-score for CXCR4+ tumor was not prognostic of progression-free survival (PFS) or overall survival (OS). Baseline CXCR4+ CTCs ≥7% was prognostic of shorter PFS. CTCs ≥6 at baseline and cycle 2, day 1 were prognostic of shorter PFS and OS. None of the biomarkers at their respective optimum cutoffs was predictive of treatment response of LY2510924 plus CE versus CE. Conclusions In patients with ED-SCLC, baseline CXCR4 expression in tumor tissue was not prognostic of survival or predictive of LY2510924 treatment response. Baseline CXCR4+ CTCs ≥7% was prognostic of shorter PFS. CTC count ≥6 at baseline and after 1 cycle of treatment were prognostic of shorter PFS and OS.
Keywords: CXCR4 expression; Carboplatin-etoposide; Circulating tumor cells; LY2510924; Small cell lung cancer.
Conflict of interest statement
Disclosure of potential conflicts of interest
J.R. Stille, S.B. Yan, S. Roberson, J. Polzer, E. Raddad, V.L. Peek, S.R. Wijayawardana, and S.L. Um are employees and shareholders of Eli Lilly and Company.
S. Gross, M.C. Connelly, C. Morano, M. Repollet, R. Sanders, K. Baeten, and D. D’Haese are employees of Janssen Diagnostics and shareholders of Johnson and Johnson Company.
S. Gross and C. Morano receive royalties from Johnson and Johnson Company.
D.R. Spigel receives no compensation as an advisor to Bristol-Myers Squibb, Genentech/Roche, Novartis, and Pfizer and serves on a data safety monitoring board for Merck.
No conflict of interest was reported by R. Salgia, M. McCleod, R.W. Weaver, or A. Flynt.
Funding
This study was funded by Eli Lilly and Company.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. The informed consent form was signed by the patient and physician prior to study procedures in accordance with International Conference on Harmonization guidelines on Good Clinical Practice.
Figures


References
-
- American Cancer Society (2016) Lung cancer (small cell) http://www.cancer.org/acs/groups/cid/documents/webcontent/003116-pdf.pdf. Accessed 11 Aug 2016
-
- Reck M, Thatcher N, Smit EF, et al. Baseline quality of life and performance status as prognostic factors in patients with extensive-stage disease small cell lung cancer treated with pemetrexed plus carboplatin vs. etoposide plus carboplatin. Lung Cancer. 2012;78:276–281. doi: 10.1016/j.lungcan.2012.09.002. - DOI - PubMed
-
- American Cancer Society . Cancer facts & figures 2016. Atlanta: American Cancer Society; 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

