A versatile method for the preparation of particle-loaded microbubbles for multimodality imaging and targeted drug delivery
- PMID: 28299722
- PMCID: PMC5830459
- DOI: 10.1007/s13346-017-0366-7
A versatile method for the preparation of particle-loaded microbubbles for multimodality imaging and targeted drug delivery
Abstract
Microbubbles are currently in clinical use as ultrasound contrast agents and under active investigation as mediators of ultrasound therapy. To improve the theranostic potential of microbubbles, nanoparticles can be attached to the bubble shell for imaging, targeting and/or enhancement of acoustic response. Existing methods for fabricating particle-loaded bubbles, however, require the use of polymers, oil layers or chemical reactions for particle incorporation; embed/attach the particles that can reduce echogenicity; impair biocompatibility; and/or involve multiple processing steps. Here, we describe a simple method to embed nanoparticles in a phospholipid-coated microbubble formulation that overcomes these limitations. Magnetic nanoparticles are used to demonstrate the method with a range of different microbubble formulations. The size distribution and yield of microbubbles are shown to be unaffected by the addition of the particles. We further show that the microbubbles can be retained against flow using a permanent magnet, can be visualised by both ultrasound and magnetic resonance imaging (MRI) and can be used to transfect SH-SY5Y cells with fluorescent small interfering RNA under the application of a magnetic field and ultrasound field.
Keywords: Contrast agents; Drug delivery; Magnetic; Microbubbles; Targeting; Ultrasound.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
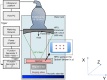

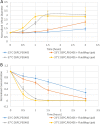
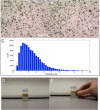
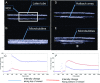



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

