Convection-Enhanced Delivery
- PMID: 28299724
- PMCID: PMC5398992
- DOI: 10.1007/s13311-017-0520-4
Convection-Enhanced Delivery
Abstract
Convection-enhanced delivery (CED) is a promising technique that generates a pressure gradient at the tip of an infusion catheter to deliver therapeutics directly through the interstitial spaces of the central nervous system. It addresses and offers solutions to many limitations of conventional techniques, allowing for delivery past the blood-brain barrier in a targeted and safe manner that can achieve therapeutic drug concentrations. CED is a broadly applicable technique that can be used to deliver a variety of therapeutic compounds for a diversity of diseases, including malignant gliomas, Parkinson's disease, and Alzheimer's disease. While a number of technological advances have been made since its development in the early 1990s, clinical trials with CED have been largely unsuccessful, and have illuminated a number of parameters that still need to be addressed for successful clinical application. This review addresses the physical principles behind CED, limitations in the technique, as well as means to overcome these limitations, clinical trials that have been performed, and future developments.
Keywords: Blood–brain barrier; Central nervous system; Convection-enhanced delivery; Drug delivery; Malignant gliomas; Technique.
Figures
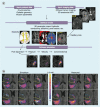
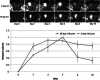
References
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Blasberg RG, Patlak C, Fenstermacher JD. Intrathecal chemotherapy: brain tissue profiles after ventriculocisternal perfusion. J Pharmacol Exp Ther. 1975;195:73–83. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

