Electroacupuncture Promotes Central Nervous System-Dependent Release of Mesenchymal Stem Cells
- PMID: 28299842
- PMCID: PMC5530374
- DOI: 10.1002/stem.2613
Electroacupuncture Promotes Central Nervous System-Dependent Release of Mesenchymal Stem Cells
Abstract
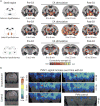
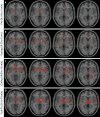
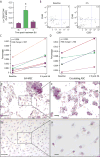
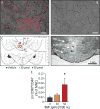
Electroacupuncture (EA) performed in rats and humans using limb acupuncture sites, LI-4 and LI-11, and GV-14 and GV-20 (humans) and Bai-hui (rats) increased functional connectivity between the anterior hypothalamus and the amygdala and mobilized mesenchymal stem cells (MSCs) into the systemic circulation. In human subjects, the source of the MSC was found to be primarily adipose tissue, whereas in rodents the tissue sources were considered more heterogeneous. Pharmacological disinhibition of rat hypothalamus enhanced sympathetic nervous system (SNS) activation and similarly resulted in a release of MSC into the circulation. EA-mediated SNS activation was further supported by browning of white adipose tissue in rats. EA treatment of rats undergoing partial rupture of the Achilles tendon resulted in reduced mechanical hyperalgesia, increased serum interleukin-10 levels and tendon remodeling, effects blocked in propranolol-treated rodents. To distinguish the afferent role of the peripheral nervous system, phosphoinositide-interacting regulator of transient receptor potential channels (Pirt)-GCaMP3 (genetically encoded calcium sensor) mice were treated with EA acupuncture points, ST-36 and LIV-3, and GV-14 and Bai-hui and resulted in a rapid activation of primary sensory neurons. EA activated sensory ganglia and SNS centers to mediate the release of MSC that can enhance tissue repair, increase anti-inflammatory cytokine production and provide pronounced analgesic relief. Stem Cells 2017;35:1303-1315.
Keywords: Adult stem cells; Mesenchymal stem cells; Nervous system; Neurones.
© 2017 AlphaMed Press.
Figures

References
-
- Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85:355–375. - PubMed
-
- Urano K, Ogasawara S. A fundamental study on acupuncture points phenomena of dog body. Kitasato Arch Exp Med. 1978;51:95–109. - PubMed
-
- Gunn CC, Ditchburn FG, King MH, et al. Acupuncture loci: a proposal for their classification according to their relationship to known neural structures. Am J Chin Med (Gard City N Y) 1976;4:183–195. - PubMed
-
- Kim SK, Bae H. Acupuncture and immune modulation. Auton Neurosci. 2010;157:38–41. - PubMed
MeSH terms
Substances
Grants and funding
- K08 DK110429/DK/NIDDK NIH HHS/United States
- R01 HL109602/HL/NHLBI NIH HHS/United States
- R01 EY012601/EY/NEI NIH HHS/United States
- R01 EY023629/EY/NEI NIH HHS/United States
- U54 DK106846/DK/NIDDK NIH HHS/United States
- R01 EY025383/EY/NEI NIH HHS/United States
- R01 HL110170/HL/NHLBI NIH HHS/United States
- I01 BX002209/BX/BLRD VA/United States
- R01 DK090730/DK/NIDDK NIH HHS/United States
- R01 EY007739/EY/NEI NIH HHS/United States
- R01 NS054791/NS/NINDS NIH HHS/United States
- R25 GM109432/GM/NIGMS NIH HHS/United States
- P30 CA082709/CA/NCI NIH HHS/United States
- UL1 TR001427/TR/NCATS NIH HHS/United States
- R01 DK100905/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

