Chromatin modification contributes to the expression divergence of three TaGS2 homoeologs in hexaploid wheat
- PMID: 28300215
- PMCID: PMC5353557
- DOI: 10.1038/srep44677
Chromatin modification contributes to the expression divergence of three TaGS2 homoeologs in hexaploid wheat
Abstract
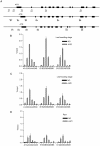
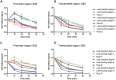
Plastic glutamine synthetase (GS2) is responsible for ammonium assimilation. The reason that TaGS2 homoeologs in hexaploid wheat experience different selection pressures in the breeding process remains unclear. TaGS2 were minimally expressed in roots but predominantly expressed in leaves, and TaGS2-B had higher expression than TaGS2-A and TaGS2-D. ChIP assays revealed that the activation of TaGS2-B expression in leaves was correlated with increased H3K4 trimethylation. The transcriptional silencing of TaGS2 in roots was correlated with greater cytosine methylation and less H3K4 trimethylation. Micrococcal nuclease and DNase I accessibility experiments indicated that the promoter region was more resistant to digestion in roots than leaves, which indicated that the closed nucleosome conformation of the promoter region was important to the transcription initiation for the spatial-temporal expression of TaGS2. In contrast, the transcribed regions possess different nuclease accessibilities of three TaGS2 homoeologs in the same tissue, suggesting that nucleosome conformation of the transcribed region was part of the fine adjustment of TaGS2 homoeologs. This study provides evidence that histone modification, DNA methylation and nuclease accessibility coordinated the control of the transcription of TaGS2 homoeologs. Our results provided important evidence that TaGS2-B experienced the strongest selection pressures during the breeding process.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Epigenetic modification contributes to the expression divergence of three TaEXPA1 homoeologs in hexaploid wheat (Triticum aestivum).New Phytol. 2013 Mar;197(4):1344-1352. doi: 10.1111/nph.12131. Epub 2013 Jan 29. New Phytol. 2013. PMID: 23360546
-
Genetic and epigenetic alteration among three homoeologous genes of a class E MADS box gene in hexaploid wheat.Plant Cell. 2007 Jun;19(6):1723-37. doi: 10.1105/tpc.107.051813. Epub 2007 Jun 22. Plant Cell. 2007. PMID: 17586655 Free PMC article.
-
An optimised chromatin immunoprecipitation (ChIP) method for starchy leaves of Nicotiana benthamiana to study histone modifications of an allotetraploid plant.Mol Biol Rep. 2020 Dec;47(12):9499-9509. doi: 10.1007/s11033-020-06013-1. Epub 2020 Nov 25. Mol Biol Rep. 2020. PMID: 33237398 Free PMC article.
-
Evolution of Deeper Rooting 1-like homoeologs in wheat entails the C-terminus mutations as well as gain and loss of auxin response elements.PLoS One. 2019 Apr 4;14(4):e0214145. doi: 10.1371/journal.pone.0214145. eCollection 2019. PLoS One. 2019. PMID: 30947257 Free PMC article.
-
Asymmetrical changes of gene expression, small RNAs and chromatin in two resynthesized wheat allotetraploids.Plant J. 2018 Mar;93(5):828-842. doi: 10.1111/tpj.13805. Epub 2018 Jan 27. Plant J. 2018. PMID: 29265531
Cited by
-
H3K4/K9 acetylation and Lr28-mediated expression of six leaf rust responsive genes in wheat (Triticum aestivum).Mol Genet Genomics. 2019 Feb;294(1):227-241. doi: 10.1007/s00438-018-1500-z. Epub 2018 Oct 8. Mol Genet Genomics. 2019. PMID: 30298213
-
Overexpression of TaPIP1A enhances drought and salt stress tolerance in Arabidopsis: cross-species conservation and molecular dynamics.Front Plant Sci. 2025 Jun 2;15:1425700. doi: 10.3389/fpls.2024.1425700. eCollection 2024. Front Plant Sci. 2025. PMID: 40528925 Free PMC article.
-
Homoeolog expression bias in allopolyploid oleaginous marine diatom Fistulifera solaris.BMC Genomics. 2018 May 4;19(1):330. doi: 10.1186/s12864-018-4691-0. BMC Genomics. 2018. PMID: 29728068 Free PMC article.
-
Development and marker-trait relationships of functional markers for glutamine synthetase GS1 and GS2 homoeogenes in bread wheat.Mol Breed. 2023 Jan 19;43(2):8. doi: 10.1007/s11032-022-01354-0. eCollection 2023 Feb. Mol Breed. 2023. PMID: 37309364 Free PMC article.
-
Alternative Splicing of TaGS3 Differentially Regulates Grain Weight and Size in Bread Wheat.Int J Mol Sci. 2021 Oct 28;22(21):11692. doi: 10.3390/ijms222111692. Int J Mol Sci. 2021. PMID: 34769129 Free PMC article.
References
-
- Miflin B. J. & Habash D. Z. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. Journal of Experimental Botany 53, 979–987 (2002). - PubMed
-
- Lea P. J. & Miflin B. J. Nitrogen assimilation and its relevance to crop improvement. Nitrogen metabolism in plants in the post-genomic era. Chichester, UK: Wiley-Blackwell, 1–40 (2011).
-
- Bernard S. M. et al.. Gene expression, cellular localisation and function of glutamine synthetase isozymes in wheat (Triticum aestivum L.). Plant molecular biology 67, 89–105 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

