Synthesis and Evaluation of New Pyrazoline Derivatives as Potential Anticancer Agents in HepG-2 Cell Line
- PMID: 28300751
- PMCID: PMC6155299
- DOI: 10.3390/molecules22030467
Synthesis and Evaluation of New Pyrazoline Derivatives as Potential Anticancer Agents in HepG-2 Cell Line
Abstract
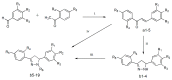
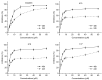
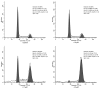
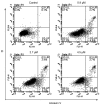
Cancer is a major public health concern worldwide. Adverse effects of cancer treatments still compromise patients' quality of life. To identify new potential anticancer agents, a series of novel pyrazoline derivatives were synthesized and evaluated for cytotoxic effects on HepG-2 (human liver hepatocellular carcinoma cell line) and primary hepatocytes. Compound structures were confirmed by ¹H-NMR, mass spectrometry, and infrared imaging. An in vitro assay demonstrated that several compounds exerted cytotoxicity in the micromolar range. Benzo[b]thiophen-2-yl-[5-(4-hydroxy-3,5-dimethoxy-phenyl)-3-(2-hydroxy-phenyl)-4,5-dihydo-pyrazol-1-yl]-methanone (b17) was the most effective anticancer agent against HepG-2 cells owing to its notable inhibitory effect on HepG-2 with an IC50 value of 3.57 µM when compared with cisplatin (IC50 = 8.45 µM) and low cytotoxicity against primary hepatocytes. Cell cycle analysis and apoptosis/necrosis evaluation using this compound revealed that b17 notably arrested HepG-2 cells in the G₂/M phase and induced HepG-2 cells apoptosis. Our findings indicate that compound b17 may be a promising anticancer drug candidate.
Keywords: HepG-2 cells; anticancer activity; apoptosis; pyrazoline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

