Cystatin SN inhibits auranofin-induced cell death by autophagic induction and ROS regulation via glutathione reductase activity in colorectal cancer
- PMID: 28300829
- PMCID: PMC5386512
- DOI: 10.1038/cddis.2017.100
Cystatin SN inhibits auranofin-induced cell death by autophagic induction and ROS regulation via glutathione reductase activity in colorectal cancer
Erratum in
-
Cystatin SN inhibits auranofin-induced cell death by autophagic induction and ROS regulation via glutathione reductase activity in colorectal cancer.Cell Death Dis. 2017 Sep 14;8(9):e3053. doi: 10.1038/cddis.2017.446. Cell Death Dis. 2017. PMID: 28906491 Free PMC article.
Abstract
Cystatin SN (CST1) is a specific inhibitor belonging to the cystatin superfamily that controls the proteolytic activities of cysteine proteases such as cathepsins. Our previous study showed that high CST1 expression enhances tumor metastasis and invasiveness in colorectal cancer. Recently, auranofin (AF), a gold(I)-containing thioredoxin reductase 1 (TrxR1) inhibitor, has been used clinically to treat rheumatoid arthritis. AF is a proteasome-associated deubiquitinase inhibitor and can act as an anti-tumor agent. In this study, we investigated whether CST1 expression induces autophagy and tumor cell survival. We also investigated the therapeutic effects of AF as an anti-tumor agent in colorectal cancer (CRC) cells. We found that CRC cells expressing high levels of CST1 undergo increased autophagy and exhibit chemotherapeutic resistance to AF-induced cell death, while those expressing low levels of CST1 are sensitive to AF. We also observed that knockdown of CST1 in high-CST1 CRC cells using CST1-specific small interfering RNAs attenuated autophagic activation and restored AF-induced cell mortality. Conversely, the overexpression of CST1 increased autophagy and viability in cells expressing low levels of CST1. Interestingly, high expression of CST1 attenuates AF-induced cell death by inhibiting intracellular reactive oxygen species (ROS) generation, as demonstrated by the fact that the blockage of ROS production reversed AF-induced cell death in CRC cells. In addition, upregulation of CST1 expression increased cellular glutathione reductase (GR) activity, reducing the cellular redox state and inducing autophagy in AF-treated CRC cells. These results suggest that high CST1 expression may be involved in autophagic induction and protects from AF-induced cell death by inhibition of ROS generation through the regulation of GR activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
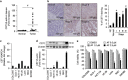

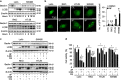



References
-
- Lah TT, Babnik J, Schiffmann E, Turk V, Skaleric U. Cysteine proteinases and inhibitors in inflammation: their role in periodontal disease. J Periodontol 1993; 64: 485–491. - PubMed
-
- Koblinski JE, AhramM, Sloane BF. Unraveling the role of proteases in cancer. Clin Chim Acta 2000; 291: 113–135. - PubMed
-
- Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer 2006; 6: 764–775. - PubMed
-
- Barrett AJ. The cystatins: a diverse superfamily of cysteine peptidase inhibitors. Biomed Biochim Acta 1986; 45: 1363–1374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

