Clinical development of anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma
- PMID: 28301076
- PMCID: PMC5480083
- DOI: 10.1111/cas.13239
Clinical development of anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma
Abstract
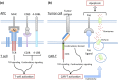
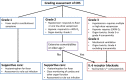
B-cell non-Hodgkin lymphoma (B-NHL) is the most frequent hematological malignancy. Although refined chemotherapy regimens and several new therapeutics including rituximab, a chimeric anti-CD20 monoclonal antibody, have improved its prognosis in recent decades, there are still a substantial number of patients with chemorefractory B-NHL. Anti-CD19 chimeric antigen receptor (CAR) T-cell therapy is expected to be an effective adoptive cell treatment and has the potential to overcome the chemorefractoriness of B-cell leukemia and lymphoma. Recently, several clinical trials have shown remarkable efficacy of anti-CD19 CAR T-cell therapy, not only in B-acute lymphoblastic leukemia but also in B-NHL. Nonetheless, there are several challenges to overcome before introduction into clinical practice, such as: (i) further refinement of the manufacturing process, (ii) further improvement of efficacy, (iii) finding the optimal infusion cell dose, (iv) optimization of lymphocyte-depleting chemotherapy, (v) identification of the best CAR structure, and (vi) optimization of toxicity management including cytokine release syndrome, neurologic toxicity, and on-target off-tumor toxicity. Several ways to solve these problems are currently under study. In this review, we describe the updated clinical data regarding anti-CD19 CAR T-cell therapy, with a focus on B-NHL, and discuss the clinical implications and perspectives of CAR T-cell therapy.
Keywords: Adoptive cell therapy; B-cell non-Hodgkin lymphoma; CAR T; CD19; chimeric antigen receptor.
© 2017 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

