Designing Hydrogels for On-Demand Therapy
- PMID: 28301139
- PMCID: PMC6527116
- DOI: 10.1021/acs.accounts.6b00536
Designing Hydrogels for On-Demand Therapy
Abstract
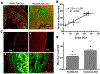
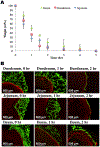
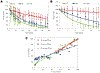
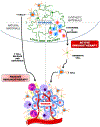
Systemic administration of therapeutic agents has been the preferred approach to treat most pathological conditions, in particular for cancer therapy. This treatment modality is associated with side effects, off-target accumulation, toxicity, and rapid renal and hepatic clearance. Multiple efforts have focused on incorporating targeting moieties into systemic therapeutic vehicles to enhance retention and minimize clearance and side effects. However, only a small percentage of the nanoparticles administered systemically accumulate at the tumor site, leading to poor therapeutic efficacy. This has prompted researchers to call the status quo treatment regimen into question and to leverage new delivery materials and alternative administration routes to improve therapeutic outcomes. Recent approaches rely on the use of local delivery platforms that circumvent the hurdles of systemic delivery. Local administration allows delivery of higher "effective" doses while enhancing therapeutic molecules' stability, minimizing side effects, clearance, and accumulation in the liver and kidneys following systemic administration. Hydrogels have proven to be highly biocompatible materials that allow for versatile design to afford sensing and therapy at the same time. Hydrogels' chemical and physical versatility can be exploited to attain disease-triggered in situ assembly and hydrogel programmed degradation and consequent drug release, and hydrogels can also serve as a biocompatible depot for local delivery of stimuli-responsive therapeutic cargo. We will focus this Account on the hydrogel platform that we have developed in our lab, based on dendrimer amine and dextran aldehyde. This hydrogel is disease-responsive and capable of sensing the microenvironment and reacting in a graded manner to diverse pathologies to render different properties, including tissue adhesion, biocompatibility, hydrogel degradation, and embedded drug release profile. We also studied the degradation kinetics of our stimuli-responsive materials in vivo and analyzed the in vitro conditions under which in vitro-in vivo correlation is attained. Identifying key parameters in the in vivo microenvironment under healthy and disease conditions was key to attaining that correlation. The adhesive capacity of our dendrimer-dextran hydrogel makes it optimal for localized and sustained release of embedded drugs. We demonstrated that it affords the delivery of a range of therapeutics to combat cancer, including nucleic acids, small molecules, and antibody drugs. As a depot for local delivery, it allows a high dose of active biomolecules to be delivered directly at the tumor site. Immunotherapy, a recently blooming area in cancer therapy, may exploit stimuli-responsive hydrogels to impart systemic effects following localized therapy. Local delivery would enable release of the proper drug dose and improve drug bioavailability where needed at the same time creating memory and exerting the therapeutic effect systemically. This Account highlights our perspective on how local and systemic therapies provided by stimuli-responsive hydrogels should be used to impart more precise, long-lasting, and potent therapeutic outcomes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Li KCP; Pandit SD; Guccione S; Bednarski MD Molecular Imaging applications in nanomedicine. Biomed. Microdevices 2004, 6, 113–116. - PubMed
-
- Devadasu VR; Bhardwaj V; Kumar MNVR Can Controversial Nanotechnology Promise Drug Delivery? Chem. Rev 2013, 113, 1686–1735. - PubMed
-
- Chithrani DB Nanoparticles for improved therapeutics and imaging in cancer therapy. Recent Pat. Nanotechnol 2010, 4, 171–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

