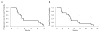A Phase II Study of Ganetespib as Second-line or Third-line Therapy for Metastatic Pancreatic Cancer
- PMID: 28301350
- PMCID: PMC5599313
- DOI: 10.1097/COC.0000000000000377
A Phase II Study of Ganetespib as Second-line or Third-line Therapy for Metastatic Pancreatic Cancer
Abstract
Objectives: Heat shock protein 90 regulates multiple signaling proteins involved in key pathways of pancreatic cancer pathogenesis. Ganetespib binds to heat shock protein 90 and interferes with its binding to client proteins thus leading to inactivation and degradation of the signaling proteins that promote cancer progression. This phase II study was designed to evaluate the efficacy of ganetespib in patients with refractory metastatic pancreatic cancer (rMPC).
Methods: Patients with rMPC received 175 mg/m ganetespib intravenously once weekly for 3 weeks in 4-week cycles. Primary endpoint was disease control rate at 8 weeks, with a goal of 70%. Secondary endpoints were progression-free survival, overall survival, and safety. Simon's 2-stage design was used to assess futility and efficacy. Ganetespib was considered inactive if ≤8 patients among the first 15 treated had disease control after 8 weeks of treatment.
Results: Fourteen patients were treated on study. Grade 3 treatment-related toxicities were diarrhea, abdominal pain, fatigue, nausea, vomiting, and hyponatremia. Disease control rate at 8 weeks was 28.6%, and median progression-free survival and overall survival were 1.58 months and 4.57 months, respectively. Early stopping rules for lack of clinical efficacy led to study closure.
Conclusions: Single-agent ganetespib was tolerable with only modest disease control in rMPC. This disease is resistant to chemotherapy, and given the emerging data in lung and rectal cancers, as well as in pancreatic cancer cell lines, suggesting improved activity of ganetespib in combination with cytotoxic agents, studies combining this agent with chemotherapy in rMPC are more likely to yield success.
Conflict of interest statement
Conflicts of Interest: DBC has served as an advisory board consultant for Merrimack and has institutional research funding from Synta, Incyte, Celgene, Hoffman-LaRoxhe, EMD-Serono, and Oncolytics Biotech. LWG has served as a consultant for Celgene and has institutional research funding from Astellas Pharma, Pfizer, Onxy, SunPharma, Lilly, and Bristol-Myers Squibb. JB has served as a consultant for Celgene, Genentech, Aduro, Boston Biomedical, Janssen, Cornerstone, Symphogen, and Bayer and has institutional research funding from Genentech, Abbvie, Taiho, Bayer, 5Prime, Phoenix, Incyte, and Vertex. EC has served on advisory boards for Castle Biosciences, Taiho, EMD-Serono, Amgen, Lilly, Advaxis, Bayer and Merrimack. For the remaining authors, none were declared.
Figures
References
-
- Miller KD, Siegel RL, Lin CC, et al. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016 Epub ahead of print. - PubMed
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA: a cancer journal for clinicians. 2008;58:71–96. - PubMed
-
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. - PubMed
-
- Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol. 2014;32:2423–9. - PubMed
-
- Yanagimoto H, Satoi S, Sho M, et al. Phase I study assessing the feasibility of the triple combination chemotherapy of SOXIRI (S-1/oxaliplatin/irinotecan) in patients with unresectable pancreatic ductal adenocarcinoma. Cancer Chemother Pharmacol. 2016;77:35–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical