The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine
- PMID: 28301400
- PMCID: PMC5359791
- DOI: 10.1097/j.pain.0000000000000831
The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine
Abstract
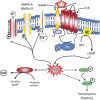
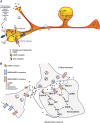
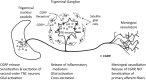
Calcitonin gene-related peptide (CGRP) is a 37-amino acid peptide found primarily in the C and Aδ sensory fibers arising from the dorsal root and trigeminal ganglia, as well as the central nervous system. Calcitonin gene-related peptide was found to play important roles in cardiovascular, digestive, and sensory functions. Although the vasodilatory properties of CGRP are well documented, its somatosensory function regarding modulation of neuronal sensitization and of enhanced pain has received considerable attention recently. Growing evidence indicates that CGRP plays a key role in the development of peripheral sensitization and the associated enhanced pain. Calcitonin gene-related peptide is implicated in the development of neurogenic inflammation and it is upregulated in conditions of inflammatory and neuropathic pain. It is most likely that CGRP facilitates nociceptive transmission and contributes to the development and maintenance of a sensitized, hyperresponsive state not only of the primary afferent sensory neurons but also of the second-order pain transmission neurons within the central nervous system, thus contributing to central sensitization as well. The maintenance of a sensitized neuronal condition is believed to be an important factor underlying migraine. Recent successful clinical studies have shown that blocking the function of CGRP can alleviate migraine. However, the mechanisms through which CGRP may contribute to migraine are still not fully understood. We reviewed the role of CGRP in primary afferents, the dorsal root ganglion, and in the trigeminal system as well as its role in peripheral and central sensitization and its potential contribution to pain processing and to migraine.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures

References
-
- Ablin JN, Buskila D, Clauw DJ. Biomarkers in fibromyalgia. Curr Pain Headache Rep 2009;13:343–9. - PubMed
-
- Afridi SK, Kaube H, Goadsby PJ. Glyceryl trinitrate triggers premonitory symptoms in migraineurs. PAIN 2004;110:675–80. - PubMed
-
- Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RS, Goadsby PJ. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 2005;128:932–9. - PubMed
-
- Amara SG, Arriza JL, Leff SE, Swanson LW, Evans RM, Rosenfeld MG. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science 1985;229:1094–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

