The Role of Bacillithiol in Gram-Positive Firmicutes
- PMID: 28301954
- PMCID: PMC5790435
- DOI: 10.1089/ars.2017.7057
The Role of Bacillithiol in Gram-Positive Firmicutes
Abstract
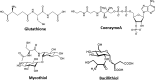
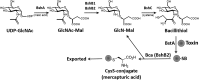
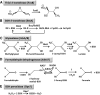
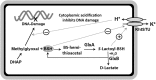
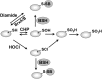
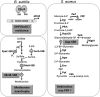
Significance: Since the discovery and structural characterization of bacillithiol (BSH), the biochemical functions of BSH-biosynthesis enzymes (BshA/B/C) and BSH-dependent detoxification enzymes (FosB, Bst, GlxA/B) have been explored in Bacillus and Staphylococcus species. It was shown that BSH plays an important role in detoxification of reactive oxygen and electrophilic species, alkylating agents, toxins, and antibiotics. Recent Advances: More recently, new functions of BSH were discovered in metal homeostasis (Zn buffering, Fe-sulfur cluster, and copper homeostasis) and virulence control in Staphylococcus aureus. Unexpectedly, strains of the S. aureus NCTC8325 lineage were identified as natural BSH-deficient mutants. Modern mass spectrometry-based approaches have revealed the global reach of protein S-bacillithiolation in Firmicutes as an important regulatory redox modification under hypochlorite stress. S-bacillithiolation of OhrR, MetE, and glyceraldehyde-3-phosphate dehydrogenase (Gap) functions, analogous to S-glutathionylation, as both a redox-regulatory device and in thiol protection under oxidative stress.
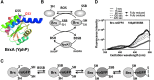
Critical issues: Although the functions of the bacilliredoxin (Brx) pathways in the reversal of S-bacillithiolations have been recently addressed, significantly more work is needed to establish the complete Brx reduction pathway, including the major enzyme(s), for reduction of oxidized BSH (BSSB) and the targets of Brx action in vivo.
Future directions: Despite the large number of identified S-bacillithiolated proteins, the physiological relevance of this redox modification was shown for only selected targets and should be a subject of future studies. In addition, many more BSH-dependent detoxification enzymes are evident from previous studies, although their roles and biochemical mechanisms require further study. This review of BSH research also pin-points these missing gaps for future research. Antioxid. Redox Signal. 28, 445-462.
Keywords: BSH biosynthesis; Bacillus subtilis; S-bacillithiolation; Staphylococcus aureus; bacilliredoxin; bacillithiol; metal homeostasis; methylglyoxal.
Figures

References
-
- Andreini C, Banci L, Bertini I, and Rosato A. Zinc through the three domains of life. J Proteome Res 5: 3173–3178, 2006 - PubMed
-
- Baichoo N, Wang T, Ye R, and Helmann JD. Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon. Mol Microbiol 45: 1613–1629, 2002 - PubMed
-
- Banci L, Bertini I, Del Conte R, Markey J, and Ruiz-Duenas FJ. Copper trafficking: the solution structure of Bacillus subtilis CopZ. Biochemistry 40: 15660–15668, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
