Characterizing and prognosticating chronic lymphocytic leukemia in the elderly: prospective evaluation on 455 patients treated in the United States
- PMID: 28302090
- PMCID: PMC5356242
- DOI: 10.1186/s12885-017-3176-x
Characterizing and prognosticating chronic lymphocytic leukemia in the elderly: prospective evaluation on 455 patients treated in the United States
Abstract
Background: Median age at diagnosis of patients with chronic lymphocytic leukemia (CLL) is > 70 years. However, the majority of clinical trials do not reflect the demographics of CLL patients treated in the community. We examined treatment patterns, outcomes, and disease-related mortality in patients ≥ 75 years with CLL (E-CLL) in a real-world setting.
Methods: The Connect® CLL registry is a multicenter, prospective observational cohort study, which enrolled 1494 adult patients between 2010-2014, at 199 US sites. Patients with CLL were enrolled within 2 months of initiating first line of therapy (LOT1) or a subsequent LOT (LOT ≥ 2). Kaplan-Meier methods were used to evaluate overall survival. CLL- and infection-related mortality were assessed using cumulative incidence functions (CIF) and cause-specific hazards. Logistic regression was used to develop a classification model.
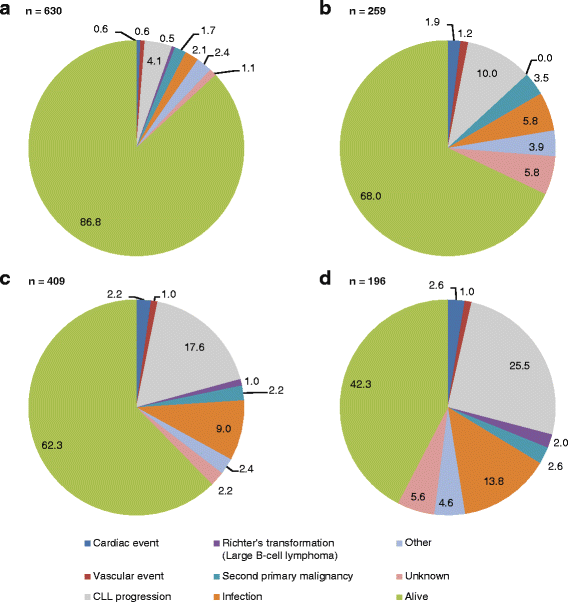
Results: A total of 455 E-CLL patients were enrolled; 259 were enrolled in LOT1 and 196 in LOT ≥ 2. E-CLL patients were more likely to receive rituximab monotherapy (19.3 vs. 8.6%; p < 0.0001) and chemotherapy-alone regimens (p < 0.0001) than younger patients. Overall and complete responses were lower in E-CLL patients than younger patients when given similar regimens. With a median follow-up of 3 years, CLL-related deaths were higher in E-CLL patients than younger patients in LOT1 (12.6 vs. 5.1% p = 0.0005) and LOT ≥ 2 (31.3 vs. 21.5%; p = 0.0277). Infection-related deaths were also higher in E-CLL patients than younger patients in LOT1 (7.4 vs. 2.7%; p = 0.0033) and in LOT ≥ 2 (16.2 vs. 11.2%; p = 0.0786). A prognostic score for E-CLL patients was developed: time from diagnosis to treatment < 3 months, enrollment therapy other than bendamustine/rituximab, and anemia, identified patients at higher risk of inferior survival. Furthermore, higher-risk patients experienced an increased risk of CLL- or infection-related death (30.6 vs 10.3%; p = 0.0006).
Conclusion: CLL- and infection-related mortality are higher in CLL patients aged ≥ 75 years than younger patients, underscoring the urgent need for alternative treatment strategies for these understudied patients.
Trial registration: The Connect CLL registry was registered at clinicaltrials.gov: NCT01081015 on March 4, 2010.
Keywords: Chemoimmunotherapy; Chronic lymphocytic leukemia; Connect® CLL registry; Elderly; Prognostic.
Figures


References
-
- Flinn IW, Neuberg DS, Grever MR, Dewald GW, Bennett JM, Paietta EM, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol. 2007;25:793–8. doi: 10.1200/JCO.2006.08.0762. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

