A dosimetric model for the heterogeneous delivery of radioactive nanoparticles In vivo: a feasibility study
- PMID: 28302144
- PMCID: PMC5356254
- DOI: 10.1186/s13014-017-0794-z
A dosimetric model for the heterogeneous delivery of radioactive nanoparticles In vivo: a feasibility study
Abstract
ᅟ: Accurate and quantitative dosimetry for internal radiation therapy can be especially challenging, given the heterogeneity of patient anatomy, tumor anatomy, and source deposition. Internal radiotherapy sources such as nanoparticles and monoclonal antibodies require high resolution imaging to accurately model the heterogeneous distribution of these sources in the tumor. The resolution of nuclear imaging modalities is not high enough to measure the heterogeneity of intratumoral nanoparticle deposition or intratumoral regions, and mathematical models do not represent the actual heterogeneous dose or dose response. To help answer questions at the interface of tumor dosimetry and tumor biology, we have modeled the actual 3-dimensional dose distribution of heterogeneously delivered radioactive nanoparticles in a tumor after systemic injection.
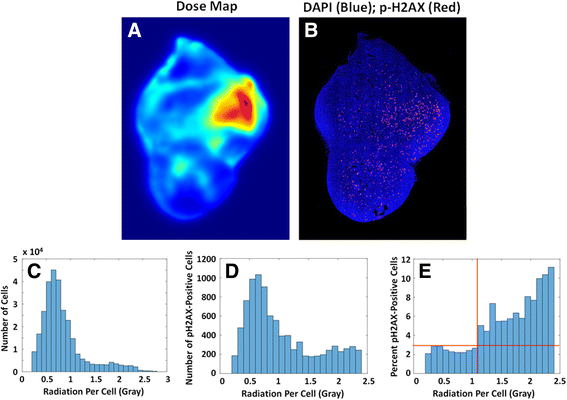
Methods: 24 h after systemic injection of dually fluorescent and radioactive nanoparticles into a tumor-bearing mouse, the tumor was cut into 342 adjacent sections and imaged to quantify the source distribution in each section. The images were stacked to generate a 3D model of source distribution, and a novel MATLAB code was employed to calculate the dose to cells on a middle section in the tumor using a low step size dose kernel.
Results: The average dose calculated by this novel 3D model compared closely with standard ways of calculating average dose, and showed a positive correlation with experimentally determined cytotoxicity in vivo. The high resolution images allowed us to determine that the dose required to initiate radiation-induced H2AX phosphorylation was approximately one Gray. The nanoparticle distribution was further used to model the dose distribution of two other radionuclides.
Conclusions: The ability of this model to quantify the absorbed dose and dose response in different intratumoral regions allows one to investigate how source deposition in different tumor areas can affect dose and cytotoxicity, as well as how characteristics of the tumor microenvironment, such as hypoxia or high stromal areas, may affect the potency of a given dose.
Keywords: Dosimetry; Heterogeneous; Internal Radiation Therapy; Nanoparticle; Tumor.
Figures





Similar articles
-
Monte Carlo simulation of radiation transport and dose deposition from locally released gold nanoparticles labeled with 111In, 177Lu or 90Y incorporated into tissue implantable depots.Phys Med Biol. 2017 Oct 27;62(22):8581-8599. doi: 10.1088/1361-6560/aa9106. Phys Med Biol. 2017. PMID: 29077574
-
Evaluating the Application of Tissue-Specific Dose Kernels Instead of Water Dose Kernels in Internal Dosimetry: A Monte Carlo Study.Cancer Biother Radiopharm. 2016 Dec;31(10):367-379. doi: 10.1089/cbr.2016.2117. Cancer Biother Radiopharm. 2016. PMID: 27996311
-
Implementation and evaluation of patient-specific three-dimensional internal dosimetry.J Nucl Med. 1997 Feb;38(2):301-8. J Nucl Med. 1997. PMID: 9025759
-
Novel Implications of Nanoparticle-Enhanced Radiotherapy and Brachytherapy: Z-Effect and Tumor Hypoxia.Metabolites. 2022 Oct 5;12(10):943. doi: 10.3390/metabo12100943. Metabolites. 2022. PMID: 36295845 Free PMC article. Review.
-
Synthetic nanoparticles for delivery of radioisotopes and radiosensitizers in cancer therapy.Cancer Nanotechnol. 2016;7(1):9. doi: 10.1186/s12645-016-0022-9. Epub 2016 Nov 16. Cancer Nanotechnol. 2016. PMID: 27909463 Free PMC article. Review.
References
-
- Gulec SA, Mesoloras G, Stabin M. Dosimetric techniques in 90Y-microsphere therapy of liver cancer: the MIRD equations for dose calculations. J Nucl Med. 2006;47(7):1209–11. - PubMed
-
- Lee YSK JS, Cho KD, Kang JH, Lim SM. Tumor dosimetry for I-131 trastuzumab therapy in a Her2+ NCI N87 xenograft mouse model using the Siemens SYMBIA E gamma camera with a pinhole collimator. J Instrum. 2015;10:P07001. doi: 10.1088/1748-0221/10/07/P07001. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

