Balanced crystalloids versus saline in the intensive care unit: study protocol for a cluster-randomized, multiple-crossover trial
- PMID: 28302179
- PMCID: PMC5356286
- DOI: 10.1186/s13063-017-1871-1
Balanced crystalloids versus saline in the intensive care unit: study protocol for a cluster-randomized, multiple-crossover trial
Abstract
Background: Saline, the intravenous fluid most commonly administered to critically ill adults, contains a high chloride content, which may be associated with acute kidney injury and death. Whether using balanced crystalloids rather than saline decreases the risk of acute kidney injury and death among critically ill adults remains unknown.
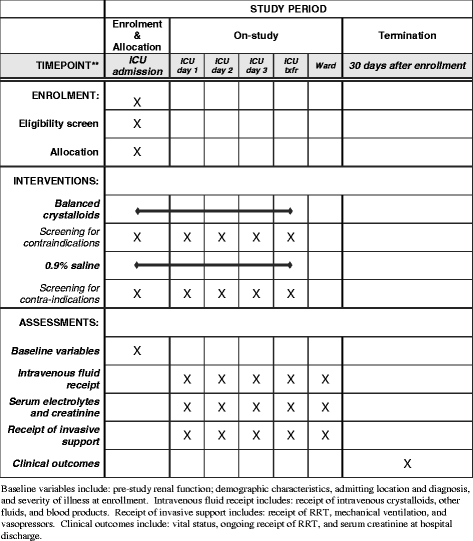
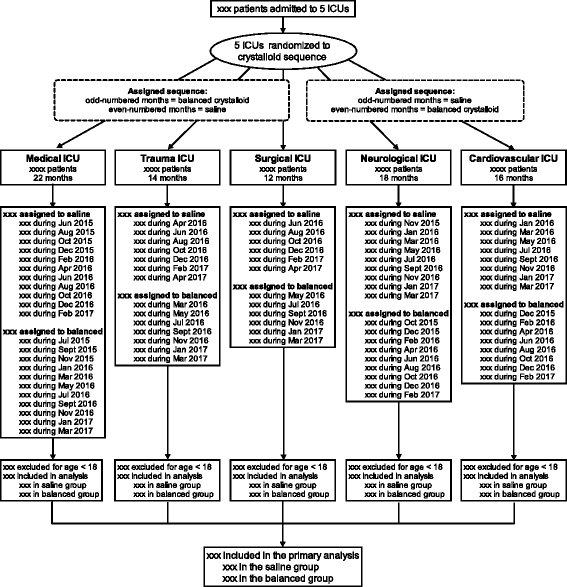
Methods: The Isotonic Solutions and Major Adverse Renal Events Trial (SMART) is a pragmatic, cluster-level allocation, cluster-level crossover trial being conducted between 1 June 2015 and 30 April 2017 in five intensive care units at Vanderbilt University Medical Center in Nashville, TN, USA. SMART compares saline (0.9% sodium chloride) with balanced crystalloids (clinician's choice of lactated Ringer's solution or Plasma-Lyte A®). Each intensive care unit is assigned to provide either saline or balanced crystalloids each month, with the assigned crystalloid alternating monthly over the course of the trial. All adults admitted to participating intensive care units during the study period are enrolled and followed until hospital discharge or 30 days after enrollment. The anticipated enrollment is approximately 14,000 patients. The primary outcome is Major Adverse Kidney Events within 30 days-the composite of in-hospital death, receipt of new renal replacement therapy, or persistent renal dysfunction (discharge creatinine ≥200% of baseline creatinine). Secondary clinical outcomes include in-hospital mortality, intensive care unit-free days, ventilator-free days, vasopressor-free days, and renal replacement therapy-free days. Secondary renal outcomes include new renal replacement therapy receipt, persistent renal dysfunction, and incidence of stage 2 or higher acute kidney injury.
Discussion: This ongoing pragmatic trial will provide the largest and most comprehensive comparison to date of clinical outcomes with saline versus balanced crystalloids among critically ill adults.
Trial registration: For logistical reasons, SMART was prospectively registered separately for the medical ICU (SMART-MED; ClinicalTrials.gov identifier: NCT02444988 ; registered on 11 May 2015; date of first patient enrollment: 1 June 2015) and the nonmedical ICUs (SMART-SURG; ClinicalTrials.gov identifier: NCT02547779 ; registered on 9 September 2015; date of first patient enrollment: 1 October 2015).
Keywords: Crystalloid; Intravenous fluids; Pragmatic trial; Renal failure; Saline.
Figures

Comment in
-
SMART: Is saline on the tightrope?Med Intensiva (Engl Ed). 2018 Aug-Sep;42(6):394-395. doi: 10.1016/j.medin.2017.12.008. Epub 2018 Feb 4. Med Intensiva (Engl Ed). 2018. PMID: 29397228 English, Spanish. No abstract available.
References
-
- Smith RJ, Reid DA, Delaney EF, Santamaria JD. Fluid therapy using a balanced crystalloid solution and acid–base stability after cardiac surgery. Crit Care Resusc. 2010;12:235–41. - PubMed
-
- Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and Plasma-Lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

