Binding mode analyses of NAP derivatives as mu opioid receptor selective ligands through docking studies and molecular dynamics simulation
- PMID: 28302509
- PMCID: PMC5395045
- DOI: 10.1016/j.bmc.2017.03.005
Binding mode analyses of NAP derivatives as mu opioid receptor selective ligands through docking studies and molecular dynamics simulation
Abstract
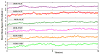
Mu opioid receptor selective antagonists are highly desirable because of their utility as pharmacological probes for receptor characterization and functional studies. Furthermore, the mu opioid receptors act as an important target in drug abuse and addiction treatment. Previously, we reported NAP as a novel lead compound with high selectivity and affinity towards the mu opioid receptor. Based on NAP, we have synthesized its derivatives and further characterized their binding affinities and selectivity towards the receptor. NMP and NGP were identified as the two most selective MOR ligands among NAP derivatives. In the present study, molecular modeling methods were applied to assess the dual binding modes of NAP derivatives, particularly on NMP and NGP, in three opioid receptors, in order to analyze the effects of structural modifications on the pyridyl ring of NAP on the binding affinity and selectivity. The results indicated that the steric hindrance, electrostatic, and hydrophobic effects caused by the substituents on the pyridyl ring of NAP contributed complimentarily on the binding affinity and selectivity of NAP derivatives to three opioid receptors. Analyses of these contributions provided insights on future design of more potent and selective mu opioid receptor ligands.
Keywords: Binding affinity; Dual binding modes; Molecular modeling; NAP derivatives; Opioid receptors; Selectivity.
Published by Elsevier Ltd.
Figures


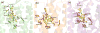

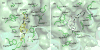
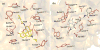

Similar articles
-
Design, Synthesis, and Biological Evaluation of the Third Generation 17-Cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4'-pyridyl)carboxamido]morphinan (NAP) Derivatives as μ/κ Opioid Receptor Dual Selective Ligands.J Med Chem. 2019 Jan 24;62(2):561-574. doi: 10.1021/acs.jmedchem.8b01158. Epub 2019 Jan 11. J Med Chem. 2019. PMID: 30608693 Free PMC article.
-
Binding mode characterization of 6α- and 6β-N-heterocyclic substituted naltrexamine derivatives via docking in opioid receptor crystal structures and site-directed mutagenesis studies: application of the 'message-address' concept in development of mu opioid receptor selective antagonists.Bioorg Med Chem. 2013 Nov 1;21(21):6405-13. doi: 10.1016/j.bmc.2013.08.042. Epub 2013 Sep 4. Bioorg Med Chem. 2013. PMID: 24055076 Free PMC article.
-
Structure selectivity relationship studies of 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4'-pyridyl)carboxamido]morphinan derivatives toward the development of the mu opioid receptor antagonists.Bioorg Med Chem Lett. 2011 Sep 15;21(18):5625-9. doi: 10.1016/j.bmcl.2011.06.135. Epub 2011 Jul 18. Bioorg Med Chem Lett. 2011. PMID: 21788135 Free PMC article.
-
17-Cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-(4'-pyridylcarboxamido)morphinan (NAP) Modulating the Mu Opioid Receptor in a Biased Fashion.ACS Chem Neurosci. 2016 Mar 16;7(3):297-304. doi: 10.1021/acschemneuro.5b00245. Epub 2016 Jan 8. ACS Chem Neurosci. 2016. PMID: 26716358 Free PMC article.
-
Diabetes Treatment: Selective Synthetic Receptor for Glucose.ChemistryOpen. 2019 Jan 24;8(1):84-86. doi: 10.1002/open.201800279. eCollection 2019 Jan. ChemistryOpen. 2019. PMID: 30693171 Free PMC article. Review.
Cited by
-
Comparison of Pharmacological Properties between the Kappa Opioid Receptor Agonist Nalfurafine and 42B, Its 3-Dehydroxy Analogue: Disconnect between in Vitro Agonist Bias and in Vivo Pharmacological Effects.ACS Chem Neurosci. 2020 Oct 7;11(19):3036-3050. doi: 10.1021/acschemneuro.0c00407. Epub 2020 Sep 24. ACS Chem Neurosci. 2020. PMID: 32897695 Free PMC article.
-
Potential Toxicity Risk Assessment and Priority Control Strategy for PAHs Metabolism and Transformation Behaviors in the Environment.Int J Environ Res Public Health. 2022 Sep 2;19(17):10972. doi: 10.3390/ijerph191710972. Int J Environ Res Public Health. 2022. PMID: 36078713 Free PMC article.
-
Structure-Activity Relationship Studies of 6α- and 6β-Indolylacetamidonaltrexamine Derivatives as Bitopic Mu Opioid Receptor Modulators and Elaboration of the "Message-Address Concept" To Comprehend Their Functional Conversion.ACS Chem Neurosci. 2019 Mar 20;10(3):1075-1090. doi: 10.1021/acschemneuro.8b00349. Epub 2018 Sep 7. ACS Chem Neurosci. 2019. PMID: 30156823 Free PMC article.
-
Characterization of 17-Cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6α-(indole-7-carboxamido)morphinan (NAN) as a Novel Opioid Receptor Modulator for Opioid Use Disorder Treatment.ACS Chem Neurosci. 2019 May 15;10(5):2518-2532. doi: 10.1021/acschemneuro.9b00038. Epub 2019 Feb 21. ACS Chem Neurosci. 2019. PMID: 30758946 Free PMC article.
-
Discovery of 6α-Thiazolylcarboxamidonaltrexamine Derivative (NTZ) as a Potent and Central Nervous System Penetrant Opioid Receptor Modulator with Drug-like Properties for Potential Treatment of Opioid Use Disorder.ACS Pharmacol Transl Sci. 2024 Dec 5;7(12):4165-4182. doi: 10.1021/acsptsci.4c00593. eCollection 2024 Dec 13. ACS Pharmacol Transl Sci. 2024. PMID: 39698260
References
-
- Fredriksson R, Lagerström MC, Lundin L-G, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. - PubMed
-
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nature reviews Drug discovery. 2006;5:993–996. - PubMed
-
- Tyndall JD, Sandilya R. GPCR agonists and antagonists in the clinic. Medicinal chemistry. 2005;1:405–421. - PubMed
-
- Harrison LM, Kastin AJ, Zadina JE. Opiate tolerance and dependence: receptors, G-proteins, and antiopiates. Peptides. 1998;19:1603–1630. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

