Bishomoisoprenoid triazole bisphosphonates as inhibitors of geranylgeranyl diphosphate synthase
- PMID: 28302510
- PMCID: PMC5450914
- DOI: 10.1016/j.bmc.2017.02.066
Bishomoisoprenoid triazole bisphosphonates as inhibitors of geranylgeranyl diphosphate synthase
Abstract
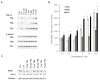
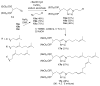
Protein geranylgeranylation reactions are dependent on the availability of geranylgeranyl diphosphate (GGDP), which serves as the isoprenoid donor. Inhibition of GGDP synthase (GGDPS) is of interest from a drug development perspective as GGDPS inhibition results in impaired protein geranylgeranylation, which in multiple myeloma, disrupts monoclonal protein trafficking and induces apoptosis. We have recently reported a series of isoprenoid triazole bisphosphonates and have demonstrated that a 3:1 mixture of homogeranyl and homoneryl isomers potently, and in a synergistic manner, inhibits GGDPS. We now present the synthesis and biological evaluation of a novel series of bishomoisoprenoid triazoles which furthers our understanding of the structure-function relationship of this class. These studies demonstrate the importance of chain length and olefin stereochemistry on inhibitory activity.
Keywords: Bishomoisoprenoids; Bisphosphonate; GGDP synthase; Inhibition; Isoprenoid biosynthesis; Triazole.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
α-Methylation enhances the potency of isoprenoid triazole bisphosphonates as geranylgeranyl diphosphate synthase inhibitors.Bioorg Med Chem. 2018 Jan 15;26(2):376-385. doi: 10.1016/j.bmc.2017.10.023. Epub 2017 Oct 19. Bioorg Med Chem. 2018. PMID: 29248353 Free PMC article.
-
Recent Advances in the Development of Mammalian Geranylgeranyl Diphosphate Synthase Inhibitors.Molecules. 2017 May 27;22(6):886. doi: 10.3390/molecules22060886. Molecules. 2017. PMID: 28555000 Free PMC article. Review.
-
Olefin Isomers of a Triazole Bisphosphonate Synergistically Inhibit Geranylgeranyl Diphosphate Synthase.Mol Pharmacol. 2017 Mar;91(3):229-236. doi: 10.1124/mol.116.107326. Epub 2017 Jan 5. Mol Pharmacol. 2017. PMID: 28057800 Free PMC article.
-
In Vivo Evaluation of Isoprenoid Triazole Bisphosphonate Inhibitors of Geranylgeranyl Diphosphate Synthase: Impact of Olefin Stereochemistry on Toxicity and Biodistribution.J Pharmacol Exp Ther. 2019 Nov;371(2):327-338. doi: 10.1124/jpet.119.258624. Epub 2019 Aug 16. J Pharmacol Exp Ther. 2019. PMID: 31420526 Free PMC article.
-
Structural Insight into Geranylgeranyl Diphosphate Synthase (GGDPS) for Cancer Therapy.Mol Cancer Ther. 2024 Jan 3;23(1):14-23. doi: 10.1158/1535-7163.MCT-23-0358. Mol Cancer Ther. 2024. PMID: 37756579 Free PMC article. Review.
Cited by
-
α-Methylation enhances the potency of isoprenoid triazole bisphosphonates as geranylgeranyl diphosphate synthase inhibitors.Bioorg Med Chem. 2018 Jan 15;26(2):376-385. doi: 10.1016/j.bmc.2017.10.023. Epub 2017 Oct 19. Bioorg Med Chem. 2018. PMID: 29248353 Free PMC article.
-
Novel benzimidazole phosphonates as potential inhibitors of protein prenylation.Bioorg Med Chem Lett. 2019 Dec 15;29(24):126757. doi: 10.1016/j.bmcl.2019.126757. Epub 2019 Oct 31. Bioorg Med Chem Lett. 2019. PMID: 31699606 Free PMC article.
-
Geranylgeranyl diphosphate synthase inhibitor and proteasome inhibitor combination therapy in multiple myeloma.Exp Hematol Oncol. 2022 Feb 9;11(1):5. doi: 10.1186/s40164-022-00261-6. Exp Hematol Oncol. 2022. PMID: 35139925 Free PMC article.
-
α-Amino bisphosphonate triazoles serve as GGDPS inhibitors.Bioorg Med Chem Lett. 2024 Apr 1;102:129659. doi: 10.1016/j.bmcl.2024.129659. Epub 2024 Feb 17. Bioorg Med Chem Lett. 2024. PMID: 38373465 Free PMC article.
-
Recent Advances in the Development of Mammalian Geranylgeranyl Diphosphate Synthase Inhibitors.Molecules. 2017 May 27;22(6):886. doi: 10.3390/molecules22060886. Molecules. 2017. PMID: 28555000 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

