Intersubunit physical couplings fostered by the left flipper domain facilitate channel opening of P2X4 receptors
- PMID: 28302727
- PMCID: PMC5418059
- DOI: 10.1074/jbc.M116.771121
Intersubunit physical couplings fostered by the left flipper domain facilitate channel opening of P2X4 receptors
Abstract
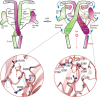
P2X receptors are ATP-gated trimeric channels with important roles in diverse pathophysiological functions. A detailed understanding of the mechanism underlying the gating process of these receptors is thus fundamentally important and may open new therapeutic avenues. The left flipper (LF) domain of the P2X receptors is a flexible loop structure, and its coordinated motions together with the dorsal fin (DF) domain are crucial for the channel gating of the P2X receptors. However, the mechanism underlying the crucial role of the LF domain in the channel gating remains obscure. Here, we propose that the ATP-induced allosteric changes of the LF domain enable it to foster intersubunit physical couplings among the DF and two lower body domains, which are pivotal for the channel gating of P2X4 receptors. Metadynamics analysis indicated that these newly established intersubunit couplings correlate well with the ATP-bound open state of the receptors. Moreover, weakening or strengthening these physical interactions with engineered intersubunit metal bridges remarkably decreased or increased the open probability of the receptors, respectively. Further disulfide cross-linking and covalent modification confirmed that the intersubunit physical couplings among the DF and two lower body domains fostered by the LF domain at the open state act as an integrated structural element that is stringently required for the channel gating of P2X4 receptors. Our observations provide new mechanistic insights into P2X receptor activation and will stimulate development of new allosteric modulators of P2X receptors.
Keywords: P2X receptors; conformational change; gating; ion channel; molecular simulations; physical couplings; protein domain; receptor structure-function; single channel recording.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


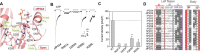








References
-
- Kellenberger S., and Grutter T. (2015) Architectural and functional similarities between trimeric ATP-gated P2X receptors and acid-sensing ion channels. J. Mol. Biol. 427, 54–66 - PubMed
-
- Khakh B. S., and North R. A. (2006) P2X receptors as cell-surface ATP sensors in health and disease. Nature 442, 527–532 - PubMed
-
- Surprenant A., and North R. A. (2009) Signaling at purinergic P2X receptors. Annu. Rev. Physiol. 71, 333–359 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

