PARP inhibitors: Synthetic lethality in the clinic
- PMID: 28302823
- PMCID: PMC6175050
- DOI: 10.1126/science.aam7344
PARP inhibitors: Synthetic lethality in the clinic
Abstract
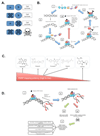
PARP inhibitors (PARPi), a cancer therapy targeting poly(ADP-ribose) polymerase, are the first clinically approved drugs designed to exploit synthetic lethality, a genetic concept proposed nearly a century ago. Tumors arising in patients who carry germline mutations in either BRCA1 or BRCA2 are sensitive to PARPi because they have a specific type of DNA repair defect. PARPi also show promising activity in more common cancers that share this repair defect. However, as with other targeted therapies, resistance to PARPi arises in advanced disease. In addition, determining the optimal use of PARPi within drug combination approaches has been challenging. Nevertheless, the preclinical discovery of PARPi synthetic lethality and the route to clinical approval provide interesting lessons for the development of other therapies. Here, we discuss current knowledge of PARP inhibitors and potential ways to maximize their clinical effectiveness.
Copyright © 2017, American Association for the Advancement of Science.
Conflict of interest statement
AA and CJL are named inventors on patents describing the use of PARP inhibitors and as such stand to gain financially as part of the ICR “Rewards to Inventors” Scheme.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

