The modification of natural products for medical use
- PMID: 28303218
- PMCID: PMC5343118
- DOI: 10.1016/j.apsb.2016.06.003
The modification of natural products for medical use
Abstract
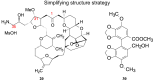
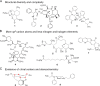
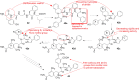
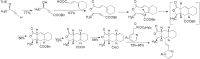
Drug innovation is characterized by painstaking molecular-level syntheses and modifications as the basic components of research and development. Similarly, natural products are chemically tailored and modified based upon their structural and biological properties. To some extent, the modification of natural products is quite different from de novo structure-based drug discovery. This review describes the general strategies and principles for the modification of natural products to drugs, as illustrated by several successful medicines that originated from natural products.
Keywords: Artemisinin; Indirubin; Multi-dimensional optimization; Natural products; Structure–acitivity reactivity; Synthesis.
Figures

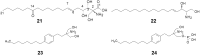



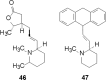
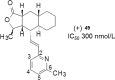

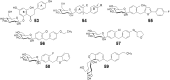

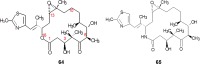
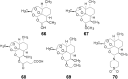
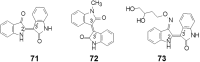
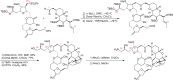
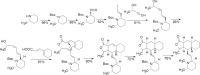
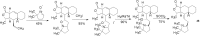
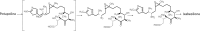



References
-
- Hopkins A.L., Groom C.R., Alex A. Ligand efficiency: a useful metric for lead selection. Drug Discov Today. 2004;9:430–431. - PubMed
-
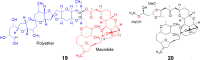
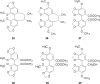
- Aicher T.D., Buszek K.R., Fang F.G., Forsyth C.J., Jung S.H., Kishi Y. Total synthesis of halichondrin B and norhalichondrin B. J Am Chem Soc. 1992;114:3162–3164.
-
- Towle M.J., Salvato K.A., Budrow J., Wels B.F., Kuznetsov G., Aalfs K.K. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61:1013–1021. - PubMed
-
- Yu M.J., Zheng W.J., Seletsky M.B. From micrograms to grams: scale-up synthesis of eribulin mesylate. Nat Prod Rep. 2013;30:1158–1164. - PubMed
-
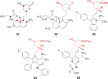
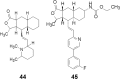
- Fujita T., Inoue K., Yamamoto S., Ikumoto T., Sasaki S., Toyama R. Fungal metabolites. Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot. 1994;47:208–215. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

