Long-Lasting Visuo-Vestibular Mismatch in Freely-Behaving Mice Reduces the Vestibulo-Ocular Reflex and Leads to Neural Changes in the Direct Vestibular Pathway
- PMID: 28303261
- PMCID: PMC5354632
- DOI: 10.1523/ENEURO.0290-16.2017
Long-Lasting Visuo-Vestibular Mismatch in Freely-Behaving Mice Reduces the Vestibulo-Ocular Reflex and Leads to Neural Changes in the Direct Vestibular Pathway
Abstract
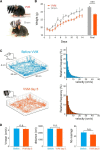
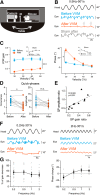
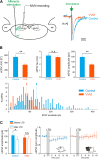
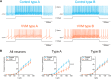
Calibration of the vestibulo-ocular reflex (VOR) depends on the presence of visual feedback. However, the cellular mechanisms associated with VOR modifications at the level of the brainstem remain largely unknown. A new protocol was designed to expose freely behaving mice to a visuo-vestibular mismatch during a 2-week period. This protocol induced a 50% reduction of the VOR. In vivo pharmacological experiments demonstrated that the VOR reduction depends on changes located outside the flocculus/paraflocculus complex. The cellular mechanisms associated with the VOR reduction were then studied in vitro on brainstem slices through a combination of vestibular afferent stimulation and patch-clamp recordings of central vestibular neurons. The evoked synaptic activity demonstrated that the efficacy of the synapses between vestibular afferents and central vestibular neurons was decreased. In addition, a long-term depression protocol failed to further decrease the synapse efficacy, suggesting that the VOR reduction might have occurred through depression-like mechanisms. Analysis of the intrinsic membrane properties of central vestibular neurons revealed that the synaptic changes were supplemented by a decrease in the spontaneous discharge and excitability of a subpopulation of neurons. Our results provide evidence that a long-lasting visuo-vestibular mismatch leads to changes in synaptic transmission and intrinsic properties of central vestibular neurons in the direct VOR pathway. Overall, these results open new avenues for future studies on visual and vestibular interactions conducted in vivo and in vitro.
Keywords: VOR; multisensory; neuronal excitability; reflex; synaptic plasticity; vestibular neurons.
Figures


References
-
- Albus JS (1971) A theory of cerebellar function. Math Biosci 10:25–61. 10.1016/0025-5564(71)90051-4 - DOI
-
- Babalian A, Vibert N, Assie G, Serafin M, Mühlethaler M, Vidal PP (1997) Central vestibular networks in the guinea-pig: functional characterization in the isolated whole brain in vitro. Neuroscience 81:405–426. - PubMed
-
- Babalian AL, Vidal PP (2000) Floccular modulation of vestibuloocular pathways and cerebellum-related plasticity: an in vitro whole brain study. J Neurophysiol 84:2514–2528. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
