Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts
- PMID: 28303661
- PMCID: PMC5397334
- DOI: 10.1111/xen.12293
Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts
Abstract
Background: Genetically engineered pigs could provide a source of kidneys for clinical transplantation. The two longest kidney graft survivals reported to date have been 136 and 310 days, but graft survival >30 days has been unusual until recently.
Methods: Donor pigs (n=4) were on an α1,3-galactosyltransferase gene-knockout (GTKO)/human complement regulatory protein (CD46) background (GTKO/CD46). In addition, the pigs were transgenic for at least one human coagulation regulatory protein. Two baboons received a kidney from a six-gene pig (GroupA) and two from a three-gene pig (GroupB). Immunosuppressive therapy was identical in all four cases and consisted of anti-thymoglobulin (ATG)+anti-CD20mAb (induction) and anti-CD40mAb+rapamycin+corticosteroids (maintenance). Anti-TNF-α and anti-IL-6R mAbs were administered to reduce the inflammatory response. Baboons were followed by clinical/laboratory monitoring of immune/coagulation/inflammatory/physiological parameters. At biopsy or euthanasia, the grafts were examined by microscopy.
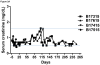
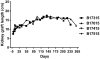
Results: The two GroupA baboons remained healthy with normal renal function >7 and >8 months, respectively, but then developed infectious complications. However, no features of a consumptive coagulopathy, eg, thrombocytopenia and reduction of fibrinogen, or of a protein-losing nephropathy were observed. There was no evidence of an elicited anti-pig antibody response, and histology of biopsies taken at approximately 4, 6, and 7 months and at necropsy showed no significant abnormalities. In contrast, both GroupB baboons developed features of a consumptive coagulopathy and required euthanasia on day 12.
Conclusions: The combination of (i) a graft from a specific six-gene genetically modified pig, (ii) an effective immunosuppressive regimen, and (iii) anti-inflammatory therapy prevented immune injury, a protein-losing nephropathy, and coagulation dysfunction for >7 months. Although the number of experiments is very limited, our impression is that expression of human endothelial protein C receptor (±CD55) in the graft is important if coagulation dysregulation is to be avoided.
Keywords: anti-IL-6R antagonist; costimulation blockade; genetically engineered; kidney; pig; xenotransplantation.
© 2017 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
David Ayares and Carol Phelps are employees of Revivicor, Inc. No other author has a conflict of interest.
Figures



















References
-
- Lambrigts D, Sachs DH, Cooper DK. Discordant organ xenotransplantation in primates: world experience and current status. Transplantation. 1998;66:547–561. - PubMed
-
- Baldan N, Rigotti P, Calabrese F, et al. Ureteral stenosis in HDAF pig-to-primate renal xenotransplantation: a phenomenon related to immunological events? Am J Transplant. 2004;4:475–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

