Sprint Interval Training Induces A Sexual Dimorphism but does not Improve Peak Bone Mass in Young and Healthy Mice
- PMID: 28303909
- PMCID: PMC5355982
- DOI: 10.1038/srep44047
Sprint Interval Training Induces A Sexual Dimorphism but does not Improve Peak Bone Mass in Young and Healthy Mice
Abstract
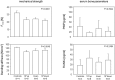
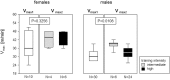
Elevated peak bone mass in early adulthood reduces the risk for osteoporotic fractures at old age. As sports participation has been correlated with elevated peak bone masses, we aimed to establish a training program that would efficiently stimulate bone accrual in healthy young mice. We combined voluntary treadmill running with sprint interval training modalities that were tailored to the individual performance limits and were of either high or intermediate intensity. Adolescent male and female STR/ort mice underwent 8 weeks of training before the hind legs were analyzed for cortical and trabecular bone parameters and biomechanical strength. Sprint interval training led to increased running speeds, confirming an efficient training. However, males and females responded differently. The males improved their running speeds in response to intermediate intensities only and accrued cortical bone at the expense of mechanical strength. High training intensities induced a significant loss of trabecular bone. The female bones showed neither adverse nor beneficial effects in response to either training intensities. Speculations about the failure to improve geometric alongside mechanical bone properties include the possibility that our training lacked sufficient axial loading, that high cardio-vascular strains adversely affect bone growth and that there are physiological limits to bone accrual.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

