A Mouse Model for Human Unstable Hemoglobin Santa Ana
- PMID: 28304246
- PMCID: PMC5157958
A Mouse Model for Human Unstable Hemoglobin Santa Ana
Abstract
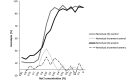
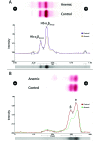
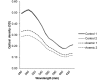
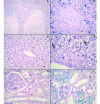
In the present study, we described the phenotype, histologic morphology, and molecular etiology of a mouse model of unstable hemoglobin Santa Ana. Hematologic evaluation of anemic mice (Anem/+) discovered after N-ethyl-N-nitrosourea mutagenesis revealed moderate anemia with intense reticulocytosis and polychromasia, followed by anisocytosis, macrocytosis, hypochromia, and intraerythrocytic inclusion and Heinz bodies. The mice also demonstrated hemoglobinuria, bilirubinemia, and erythrocytic populations with differing resistance to osmotic lysis. Splenomegaly (particularly in older mutant mice) and jaundice were apparent at necropsy. Histopathologic examination revealed dramatically increased hematopoiesis and hemosiderosis in hematopoietic organs and intracellular iron deposition in tubular renal cells. These data are characteristic of a congenital hemolytic regenerative anemia, similar to human anemias due to unstable hemoglobin. Genetic mapping assigned the affected gene to mouse chromosome 7, approximately 50 cM from the Hbb locus. The sequence of the mutant Hbb gene exhibited a T→C transversion at nucleotide 179 in Hbb-b1, leading to the substitution of proline for leucine at amino acid residue 88 and thus homologous to the genetic defect underlying Santa Ana anemia in humans.
Figures


References
-
- Benz S, Nadanaciva S, Harris D. 1995. The αβ dimer—catalytic unit of the F1 ATPase. Biochem Soc Trans 23:528S. - PubMed
-
- Biserte G, Goudemand M, Voisin D, Charlesworth D, Lorkin PA, Lehmann H. 1970. [Unstable hemoglobin during hemolytic anemia with erythrocytic inclusions and black urine. Hemoglobin found in Lille, analogous to Santa Ana β88 (F4) hemoglobin leucine–proline] Nouv Rev Fr Hematol 10:201–207. Article in French. - PubMed
-
- Fairbanks VF, Opfell RW, Burgert EO., Jr 1969. Three families with unstable hemoglobinopathies (Koln, Olmsted, and Santa Ana) causing hemolytic anemic with inclusion bodies and pigmenturia. Am J Med 46:344–359. - PubMed
-
- Gonçalves MS, Sonati MF, Kimura M, Arruda VR, Costa FF, Nechtman JF, Stoming TA. 1994. Association of Hb Santa Ana (α2β288(F4)Leu→Pro) and Hb Porto–Alegre [α2β(2)9(A6)Ser→Cys] in a Brazilian female. Hemoglobin 18:235–239. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
