Palmitate Increases β-site AβPP-Cleavage Enzyme 1 Activity and Amyloid-β Genesis by Evoking Endoplasmic Reticulum Stress and Subsequent C/EBP Homologous Protein Activation
- PMID: 28304295
- PMCID: PMC5389045
- DOI: 10.3233/JAD-161130
Palmitate Increases β-site AβPP-Cleavage Enzyme 1 Activity and Amyloid-β Genesis by Evoking Endoplasmic Reticulum Stress and Subsequent C/EBP Homologous Protein Activation
Abstract
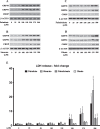
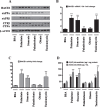
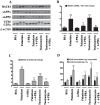
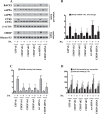
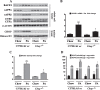
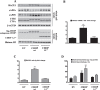
Epidemiological studies implicate diets rich in saturated free fatty acids (sFFA) as a potential risk factor for developing Alzheimer's disease (AD). In particular, high plasma levels of the sFFA palmitic acid (palmitate) were shown to inversely correlate with cognitive function. However, the cellular mechanisms by which sFFA may increase the risk for AD are not well known. Endoplasmic reticulum (ER) stress has emerged as one of the signaling pathways initiating and fostering the neurodegenerative changes in AD by increasing the aspartyl protease β-site AβPP cleaving enzyme 1 (BACE1) and amyloid-β (Aβ) genesis. In this study, we determined the extent to which palmitate increases BACE1 and Aβ levels in vitro and in vivo as well as the potential role of ER stress as cellular mechanism underlying palmitate effects. We demonstrate, in palmitate-treated SH-SY5Y neuroblastoma cells and in the hippocampi of palmitate-enriched diet-fed mice, that palmitate evokes the activation of the C/EBP Homologous Protein (CHOP), a transcription factor that is specifically responsive to ER stress. Induction of CHOP expression is associated with increased BACE1 mRNA, protein and activity levels, and subsequent enhanced amyloidogenic processing of amyloid-β protein precursor (AβPP) that culminates in a substantial increase in Aβ genesis. We further show that CHOP is an indispensable molecular mediator of palmitate-induced upregulation in BACE1 activity and Aβ genesis. Indeed, we show that Chop-/- mice and CHOP knocked-down SH-SY5Y neuroblastoma cells do not exhibit the same commensurate degree of palmitate-induced increase in BACE1 expression levels and Aβ genesis.
Keywords: Alzheimer’s disease; C/EBP homologous protein; amyloid-β; endoplasmic reticulum stress; palmitic acid; saturated free fatty acids; β-site AβPP cleaving enzyme 1.
Figures

References
-
- Haass C, Selkoe DJ (1993) Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell 75, 1039–1042. - PubMed
-
- Fukumoto H, Cheung BS, Hyman BT, Irizarry MC (2002) Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol 59, 1381–1389. - PubMed
-
- Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G (2002) Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol 51, 783–786. - PubMed
-
- Yang LB, Lindholm K, Yan R, Citron M, Xia W, Yang XL, Beach T, Sue L, Wong P, Price D, Li R, Shen Y (2003) Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med 9, 3–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

