Tardigrades Use Intrinsically Disordered Proteins to Survive Desiccation
- PMID: 28306513
- PMCID: PMC5987194
- DOI: 10.1016/j.molcel.2017.02.018
Tardigrades Use Intrinsically Disordered Proteins to Survive Desiccation
Abstract
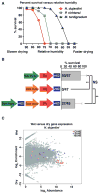
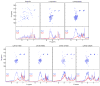
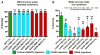
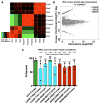
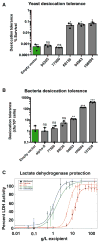
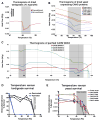
Tardigrades are microscopic animals that survive a remarkable array of stresses, including desiccation. How tardigrades survive desiccation has remained a mystery for more than 250 years. Trehalose, a disaccharide essential for several organisms to survive drying, is detected at low levels or not at all in some tardigrade species, indicating that tardigrades possess potentially novel mechanisms for surviving desiccation. Here we show that tardigrade-specific intrinsically disordered proteins (TDPs) are essential for desiccation tolerance. TDP genes are constitutively expressed at high levels or induced during desiccation in multiple tardigrade species. TDPs are required for tardigrade desiccation tolerance, and these genes are sufficient to increase desiccation tolerance when expressed in heterologous systems. TDPs form non-crystalline amorphous solids (vitrify) upon desiccation, and this vitrified state mirrors their protective capabilities. Our study identifies TDPs as functional mediators of tardigrade desiccation tolerance, expanding our knowledge of the roles and diversity of disordered proteins involved in stress tolerance.
Keywords: CAHS proteins; anhydrobiosis; cryptobiosis; desiccation tolerance; freeze tolerance; intrinsically disordered proteins; tardigrades; trehalose; vitrification; water bear.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Intrinsically Disordered Proteins Adaptively Reorganize Cellular Matter During Stress.Trends Biochem Sci. 2017 Jun;42(6):410-412. doi: 10.1016/j.tibs.2017.04.007. Epub 2017 May 6. Trends Biochem Sci. 2017. PMID: 28487210
-
Reconsidering the "glass transition" hypothesis of intrinsically unstructured CAHS proteins in desiccation tolerance of tardigrades.Mol Cell. 2021 Feb 4;81(3):409-410. doi: 10.1016/j.molcel.2020.12.007. Mol Cell. 2021. PMID: 33545053 No abstract available.
-
Water content influences the vitrified properties of CAHS proteins.Mol Cell. 2021 Feb 4;81(3):411-413. doi: 10.1016/j.molcel.2020.12.009. Mol Cell. 2021. PMID: 33545054 No abstract available.
References
-
- Altiero T, Guidetti R, Caselli V, Cesari M, Rebecchi L. Ultraviolet radiation tolerance in hydrated and desiccated eutardigrades. J Zoolog Syst Evol Res. 2011;49(Suppl 1):104–110.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

