The metabolic programming of stem cells
- PMID: 28314766
- PMCID: PMC5358754
- DOI: 10.1101/gad.293167.116
The metabolic programming of stem cells
Abstract
Advances in metabolomics have deepened our understanding of the roles that specific modes of metabolism play in programming stem cell fates. Here, we review recent metabolomic studies of stem cell metabolism that have revealed how metabolic pathways can convey changes in the extrinsic environment or their niche to program stem cell fates. The metabolic programming of stem cells represents a fine balance between the intrinsic needs of a cellular state and the constraints imposed by extrinsic conditions. A more complete understanding of these needs and constraints will afford us greater mastery over our control of stem cell fates.
Keywords: metabolomics; oxidative phosphorylation; stem cell.
© 2017 Shyh-Chang and Ng; Published by Cold Spring Harbor Laboratory Press.
Figures
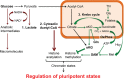

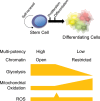
References
-
- Azuara V, Perry P, Sauer S, Spivakov M, Jørgensen HF, John RM, Gouti M, Casanova M, Warnes G, Merkenschlager M, et al. 2006. Chromatin signatures of pluripotent cell lines. Nat Cell Biol 8: 532–538. - PubMed
-
- Barbehenn EK, Wales RG, Lowry OH. 1978. Measurement of metabolites in single preimplantation embryos; a new means to study metabolic control in early embryos. J Embryol Exp Morphol 43: 29–46. - PubMed
-
- Brinster RL, Troike DE. 1979. Requirements for blastocyst development in vitro. J Anim Sci 49(Suppl 2): 26–34. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
