Precision diabetes: learning from monogenic diabetes
- PMID: 28314945
- PMCID: PMC5907633
- DOI: 10.1007/s00125-017-4226-2
Precision diabetes: learning from monogenic diabetes
Abstract
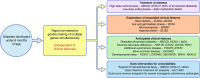
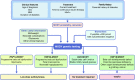
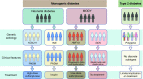
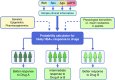
The precision medicine approach of tailoring treatment to the individual characteristics of each patient or subgroup has been a great success in monogenic diabetes subtypes, MODY and neonatal diabetes. This review examines what has led to the success of a precision medicine approach in monogenic diabetes (precision diabetes) and outlines possible implications for type 2 diabetes. For monogenic diabetes, the molecular genetics can define discrete aetiological subtypes that have profound implications on diabetes treatment and can predict future development of associated clinical features, allowing early preventative or supportive treatment. In contrast, type 2 diabetes has overlapping polygenic susceptibility and underlying aetiologies, making it difficult to define discrete clinical subtypes with a dramatic implication for treatment. The implementation of precision medicine in neonatal diabetes was simple and rapid as it was based on single clinical criteria (diagnosed <6 months of age). In contrast, in MODY it was more complex and slow because of the lack of single criteria to identify patients, but it was greatly assisted by the development of a diagnostic probability calculator and associated smartphone app. Experience in monogenic diabetes suggests that successful adoption of a precision diabetes approach in type 2 diabetes will require simple, quick, easily accessible stratification that is based on a combination of routine clinical data, rather than relying on newer technologies. Analysing existing clinical data from routine clinical practice and trials may provide early success for precision medicine in type 2 diabetes.
Keywords: GCK; HNF1A; HNF4A; KCNJ11; MODY; Maturity onset diabetes of the young; Monogenic diabetes; Neonatal diabetes; Precision diabetes; Precision medicine; Review; Type 2 diabetes.
Conflict of interest statement
Funding
This work is supported by the MASTERMIND Consortium sponsored by the Medical Research Council (MRC; MR-K005707-1) and by a Wellcome Trust Senior Investigator award given to ATH (and S. Ellard, University of Exeter Medical School, Exeter, UK [WT098395/Z/12/Z]). The work is also supported by the National Institute for Health Research (NIHR) Clinical Research Facility.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
Both authors were responsible for drafting the article and revising it critically for important intellectual content and approved the version to be published.
Figures
References
-
- National Research Council (US) Committee on A Framework for Developing a New Taxonomy of Disease (2011) Toward precision medicine: building a knowledge network for biomedical research and a new taxonomy of disease. National Academies Press, Washington, DC, USA - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

