A cellular mechanism for inverse effectiveness in multisensory integration
- PMID: 28315524
- PMCID: PMC5375642
- DOI: 10.7554/eLife.25392
A cellular mechanism for inverse effectiveness in multisensory integration
Abstract
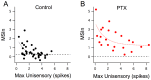
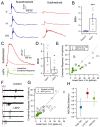
To build a coherent view of the external world, an organism needs to integrate multiple types of sensory information from different sources, a process known as multisensory integration (MSI). Previously, we showed that the temporal dependence of MSI in the optic tectum of Xenopus laevis tadpoles is mediated by the network dynamics of the recruitment of local inhibition by sensory input (Felch et al., 2016). This was one of the first cellular-level mechanisms described for MSI. Here, we expand this cellular level view of MSI by focusing on the principle of inverse effectiveness, another central feature of MSI stating that the amount of multisensory enhancement observed inversely depends on the size of unisensory responses. We show that non-linear summation of crossmodal synaptic responses, mediated by NMDA-type glutamate receptor (NMDARs) activation, form the cellular basis for inverse effectiveness, both at the cellular and behavioral levels.
Keywords: NMDA; multisensory; neural circuits; neuroscience; optic tectum; visual development; xenopus.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

References
-
- Binns KE, Salt TE. Importance of NMDA receptors for multimodal integration in the deep layers of the cat superior colliculus. Journal of Neurophysiology. 1996;75:920–930. - PubMed
-
- Deister C. imageAnalysis_gui. [56c4bcd];2016 https://github.com/cdeister/imageAnalysis_gui
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

