5-HT3A -driven green fluorescent protein delineates gustatory fibers innervating sour-responsive taste cells: A labeled line for sour taste?
- PMID: 28316078
- PMCID: PMC5933927
- DOI: 10.1002/cne.24209
5-HT3A -driven green fluorescent protein delineates gustatory fibers innervating sour-responsive taste cells: A labeled line for sour taste?
Abstract
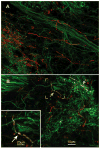
Taste buds contain multiple cell types with each type expressing receptors and transduction components for a subset of taste qualities. The sour sensing cells, Type III cells, release serotonin (5-HT) in response to the presence of sour (acidic) tastants and this released 5-HT activates 5-HT3 receptors on the gustatory nerves. We show here, using 5-HT3A GFP mice, that 5-HT3 -expressing nerve fibers preferentially contact and receive synaptic contact from Type III taste cells. Further, these 5-HT3 -expressing nerve fibers terminate in a restricted central-lateral portion of the nucleus of the solitary tract (nTS)-the same area that shows increased c-Fos expression upon presentation of a sour tastant (30 mM citric acid). This acid stimulation also evokes c-Fos in the laterally adjacent mediodorsal spinal trigeminal nucleus (DMSp5), but this trigeminal activation is not associated with the presence of 5-HT3 -expressing nerve fibers as it is in the nTS. Rather, the neuronal activation in the trigeminal complex likely is attributable to direct depolarization of acid-sensitive trigeminal nerve fibers, for example, polymodal nociceptors, rather than through taste buds. Taken together, these findings suggest that transmission of sour taste information involves communication between Type III taste cells and 5-HT3 -expressing afferent nerve fibers that project to a restricted portion of the nTS consistent with a crude mapping of taste quality information in the primary gustatory nucleus.
Keywords: chemesthesis; citric acid; serotonin receptor; solitary tract; taste bud; trigeminal.
© 2017 Wiley Periodicals, Inc.
Figures


References
-
- Archer S, Li TT, Evans AT, Britland ST, Morgan H. Cell reactions to dielectrophoretic manipulation. Biochem Biophys Res Commun. 1999;257:687–698. - PubMed
-
- Bae YC, Oh JM, Hwang SJ, Shigenaga Y, Valtschanoff JG. Expression of vanilloid receptor TRPV1 in the rat trigeminal sensory nuclei. J Comp Neurol. 2004;478:62–71. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

