Evaluation of Nanobody Conjugates and Protein Fusions as Bioanalytical Reagents
- PMID: 28316235
- PMCID: PMC5796518
- DOI: 10.1021/acs.analchem.7b00470
Evaluation of Nanobody Conjugates and Protein Fusions as Bioanalytical Reagents
Abstract
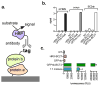
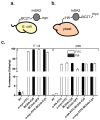
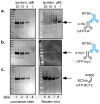
Enzyme-linked immunosorbent assay (ELISA), flow cytometry, and Western blot are common bioanalytical techniques. Successful execution traditionally requires the use of one or more commercially available antibody-small-molecule dyes or antibody-reporter protein conjugates that recognize relatively short peptide tags (<15 amino acids). However, the size of antibodies and their molecular complexity (by virtue of post-translational disulfide formation and glycosylation) typically require either expression in mammalian cells or purification from immunized mammals. The preparation and purification of chemical dye- or reporter protein-antibody conjugates is often complicated and expensive and not commonplace in academic laboratories. In response, researchers have developed comparatively simpler protein scaffolds for macromolecular recognition, which can be expressed with relative ease in E. coli and can be evolved to bind virtually any target. Nanobodies, a minimalist scaffold generated from camelid-derived heavy-chain IgGs, are one such example. A multitude of nanobodies have been evolved to recognize a diverse array of targets, including a short peptide. Here, this peptide tag (termed BC2T) and BC2 nanobody-dye conjugates or reporter protein fusions are evaluated in ELISA, flow cytometry, and Western blot experiments and compared to analogous experiments using commercially available antibody-conjugate/peptide tag pairs. Collectively, the utility and practicality of nanobody-based reagents in bioanalytical chemistry is demonstrated.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

